| |
|
The failure to stop smallpox transmission when 80% of the population was vaccinated against the virus led to a 1964 WHO expert committee
recommendation that the goal of the smallpox eradication campaign must be to vaccinate 100% of the population
(7); however, that goal was difficult if
not impossible to achieve in India. In 1973, the strategy was changed, with emphasis on surveillance to detect and then contain outbreaks of smallpox.
This strategy and its variants worked so well that by 1977 India was officially declared free of smallpox
(7).
There have also been recorded instances when smallpox disappeared even though <80% of the population was vaccinated. For example, in 1968, Sierra
Leone had the highest incidence of smallpox in the world; yet the disease disappeared in 1969 when only 66% of the population had been vaccinated
(8).
Similarly, smallpox disappeared in Mali when only 51% of the population was vaccinated
(8). In these and other West African countries, one reason that
smallpox disappeared without >80% of the susceptible population being immunized is that the eradication program shifted to a policy of focusing on
controlling outbreaks. Each outbreak was promptly investigated, and all the susceptible population surrounding the reported case(s) was vaccinated (i.e., a
"ring" vaccination policy) (8).
Rate of Transmission as Measured by Attack Rates among Susceptible Populations
Data collected from an outbreak of smallpox in Sheffield, England, >100 years ago can be used to demonstrate both the attack rate of smallpox and the
risk factors associated with infection (Table 2). The investigators found that persons with a history of vaccination or immunity (generally defined as
having a visible vaccination scar or a history of a clinical case of smallpox) had attack rates 60% to 96% lower than those of persons without a history of
vaccination (Table 2).
The attack rates among the unvaccinated "general population" are approximately 87% lower than those among the unvaccinated who lived in the same
house as a person with a previously confirmed case. In other words, the most susceptible population was unvaccinated persons who lived in close
proximity to a smallpox patient. (1) From Table
2, we can conclude that smallpox in Sheffield was not readily spread among the general population by
brief, casual encounters, such as walking down the street beside an ill person or briefly being in the same shop or business. Rather, smallpox was
primarily spread among persons living in the same house as a smallpox patient. One can only guess how crowded the average living conditions were in
the industrial town of Sheffield in the late Victorian era.
|
| |
|
|
Table 4.
Number of persons directly infected by an infectious case of smallpox
|
|
|
|
|
No. infected per infectious person |
|
|
Year and duration of |
|
|
|
| Site |
outbreak |
Total no. of casesa |
Range |
Mean |
Ref. |
|
| Erode, Tamil Nadu,
India |
1969;
2.5 months |
6 |
0 - 3 |
1 |
21 |
| Visalur, Tamil Nadu,
India |
1969;
1 month |
1 |
0 |
0 |
21 |
| Bengal, East Pakistanb |
1967;
12 months |
20c |
0-2.3 |
0.8 |
10 |
| Campo Alegre, Brazil |
1968-69;
10 months |
74 |
n/ad |
2.1 |
13 |
| Gerere hamlet, Nigeria |
1968;
4 months |
12e |
n/ae |
2e |
14 |
| Kathmandu Valley,
Nepal |
1966-67:
various |
47 |
0-7f |
2.75 |
22 |
| Chingleput district,
Madras, India |
1968 |
47g |
0-?g |
0g |
16 |
| Madras, India |
1968 |
25 |
0-4 |
0.48 |
23 |
| Bawku district, Ghana |
1967 |
66 |
0-11 |
0.9 |
24 |
| Punjab District, West
Pakistan |
1968-70 |
138h |
n/ah |
1.2h |
18 |
| Loralai District
Pakistan |
1971 |
23 |
0-9 |
2i |
25 |
| Botswana |
1973 |
30 |
0-3 |
0.78 |
26 |
| Yugoslavia |
1972 |
175 |
0-38j |
8-11j |
27 |
| Meschede Hospital,
Germany |
1970 |
20 |
0-17 |
0.95k |
28 |
| London, UK |
1961 |
3 |
0-2 |
0.66 |
29 |
| West Bromwich, UK |
1961 |
2 |
0-1 |
0.5 |
29 |
| Bradford, UK |
1961 |
14 |
0-10 |
0.9 |
29 |
| Birmingham, UK |
1962 |
1 |
0 |
0 |
29 |
| Cardiff, UK |
1962 |
47 |
0-18 |
0.97 |
29 |
| Toffo-Gare, Dahomey |
1967 |
28 |
0-4 |
0.93 |
30 |
|
|
aTotal number of cases includes the index patients who spread the disease to others.
bEast Pakistan is now called Bangladesh.
cIn the area studied, for the time reported, there were 119 cases in 30 outbreaks. However, data regarding the number of "introducers" and the number of
first-generation cases associated with those introducers were limited to 20 cases (11 introducers, 9 first-generation cases).
dThe source (13) provided only the total number of primary or coprimary cases and total number of secondary cases. Thus, only an average number of
cases per infectious person can be calculated.
eThe total number of reported cases was 62. However, the reported average was calculated from a subset of 12 cases in a single compound of 24 people
who lived in the village where the outbreak occurred. The source (14) reported the total number of generations
(6) and the total number of cases in the
compound, but not the actual cases per generation.
fAlthough the source reported 13 outbreaks resulting in 47 cases, the source of infection could be traced in only four outbreaks. Further, the source did not
report generations, only "subsequent cases," which may be a single generation or more. Thus, the upper range of 7 cases per infectious case may be an
overestimate.
gThe source reported 47 cases but only specifically identified transmission (who infected whom) of one patient admitted to an infectious disease hospital
in Madras. This patient, despite being sick at home for nearly 8 days, did not infect anyone else.
hThe source did not specify the number of index cases, although the authors reported data for 47 outbreaks, resulting in 70 first-generation and 21 second-generation cases. Our assumption that there was a single index case per outbreak maximizes the calculated average transmission rate.
iThe source reported that four second-generation cases infected eight third-generation cases. However, among these cases, the authors did not describe
who infected whom. Therefore, the average was calculated by assuming that just one of the second-generation cases infected all eight third-generation
cases. This assumption maximizes the calculated average transmission rate.
jThis is a weighted average, based on the report of 11 first-generation cases, 140 second-generation cases, and 23 third-generation cases. Thus, the average
first to second generation was 13 cases per infectious person, and the average second to third generation was 0.2 cases per infectious person. However,
since one first-generation case caused 38 second-generation cases (reputedly the largest reported number of infections known to have been caused by a
single patient) and another first generation caused 16 second-generation cases, there must have been a number of first- and second-generation cases that
did not infect any others. Removing these two first-generation cases and the second-generation case attributed to them, the weighted average becomes 8
(11 first generation, 86 second generation, and 23 third generation).
kAlthough one patient infected 17 others, only two other patients infected one case each. The other 17 patients did not transmit smallpox to others.
|
|
| |
|
|
Figure |
|
|
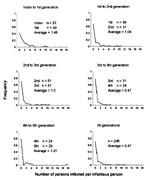 |
|
|
Click
to view enlarged image
Figure. Frequency, by generation of disease, of the number of persons infected with smallpox by an infectious person. Average refers to the mean number
of persons infected. Not all sources reported five generations of disease. In some instances, the reported outbreak was contained or died out before the
fifth generation (23-26,29,30).
|
|
Since transmission was eventually halted in all the outbreaks (Table
4), most outbreaks have an average transmission rate for the entire outbreak of <1
person infected per infectious person. A more detailed examination of the data from six of the outbreaks is presented in the
Figure, which presents the
frequency of persons infected per infectious person over time (generation of disease). The average rate of transmission per generation ranges from 0.47
persons infected per infectious person (third to fourth generation) to 1.48 (index cases to first generation) (Figure). The overall rate of transmission in the
six outbreaks was 0.47. In any given generation, there is a wide range in the number of persons infected per infectious person, ranging from zero
(occurring in all generations) to as high as 11 or even 18 (the latter occurring in the graph depicting transmission from the fourth to fifth generation).
Further evidence of the relative difficulty for one person to infect (i.e., explaining low transmission rates) is found in data representing the contacts of the
last case of naturally occurring smallpox on earth (31; Table
5). The contacts are persons who visited the patient at his home when he first developed a
fever (prodromal stage), who had contact with him after he was admitted to a hospital (but before he developed a rash), and who visited him at his home
after he was initially discharged (with a rash) with a diagnosis of chickenpox. Of the 161 persons who had contact with him, at least 12 unvaccinated
persons had "face-to-face" contact yet did not subsequently become ill with clinical cases of smallpox.
More evidence that sustained close contact is typically needed for transmission is provided by data from the 1972 Yugoslavian outbreak, in which 84 of
175 patients contracted the disease while in the hospital with a smallpox patient
(27). One patient, who spent time in three different hospitals, infected 38
people, probably a record number directly infected by a single person. Close, sustained contact in a hospital, probably through a connected ventilation
system, also permitted one patient in Meschede Hospital, Germany, to directly infect 17 others
(28).
|
| |
|
Conclusions
Although smallpox cases were recorded throughout human history until its eradication in the 1970s, remarkably few data are available that allow us to
calculate the transmission rate of smallpox. Understanding the possible transmission rate of smallpox after a deliberate release of the virus is crucial to
developing estimates of impact suitable for policy planning purposes. We therefore evaluated data that potentially measured the rate of transmission by
three possible methods.
The first, and possibly most indirect, method was to examine estimates of vaccination coverage needed to eradicate smallpox. We found, however, that
the available data do not contain sufficient information regarding the transmission rate of smallpox suitable for modeling an outbreak. Experiences from
the field appear to differ distinctly from theoretical estimates. These differences stand in contrast to the experience gained from the use of vaccines to
control rubella and measles. For these diseases, vaccination levels must be >90% for disease to be eliminated
(32,33; Table 1). The overall conclusion
from the data regarding estimates of vaccination coverage needed to eradicate smallpox is that the epidemiology of smallpox differs notably from that of
other infectious diseases (1,34; Table 1).
The second method of measuring rate of transmission was to consider data relating to the attack rates. We noted, however, that attack rate can vary by
time, population, and residence of a susceptible person in the same house as an infectious person (Tables
2, 3). We therefore conclude that the use of
attack rates derived by simply dividing the number of cases of smallpox by the total population can often be an inadequate measure of the rate of
transmission of smallpox. In the report describing the Sheffield data (Table
2), average attack rates range from 1.9% (Sheffield, 1887-88) to as low as
0.2% (Leicester, 1892-93) (9). Attack rates may differ for a variety of reasons, including prior exposure to smallpox and previous vaccination. The level
of prior vaccination and naturally acquired immunity differed from town to town. In Leicester, for example, only 50%-60% of the population had been
vaccinated at the time of the outbreak (1892) (9). Thus, in considering attack rates as a measure of rate of transmission, it is important to define both the
population of susceptible persons and their degree of contact with an infectious person (e.g., whether they live in the same house as an infectious person).
Clearly, not all susceptible persons are at equal risk. This requirement makes it very difficult to use existing data regarding attack rates to calculate an
average rate of transmission.
Given the problems associated with the first two methods of calculating a transmission rate, we must therefore rely on data that directly measure the
number of persons infected per infectious person. In almost any situation, there is likely to be a wide range in the numbers infected per infectious person
(Tables 4,5; Figure). The reason for such variability is that, despite the fact that smallpox can be transmitted by aerosolized particles
(1), it is not as
easily transmissible as, for example, measles (Table 1). Some form of sustained face-to-face contact is needed to ensure transmission (Table
5). If such
close contact is a typical (but not necessarily sole) requirement for transmission, then the data in
Tables 2 and 3 can be readily explained.
Despite strong evidence that one person can infect many others, available data suggest that the average rate of transmission is <2 persons infected per
infectious person (Table 4; Figure). Given the large percentage of the population in the United States that is now susceptible (i.e., never exposed to or
vaccinated against smallpox), the average transmission rate following a deliberate release of smallpox might be >2. Unfortunately, the probability that the
average transmission rate will be >2 cannot be demonstrated reliably. Thus, in our model, we examine the impact of three rates of transmission: 2, 3, and
5 persons infected per infectious. Our data suggest that the lowest rate (2 persons infected per infectious person) is the most accurate representation of
previous transmission rates.
References
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. Geneva: World Health Organization; 1988.
- Rao AR. Smallpox. Bombay: The Kothari Book Depot, 1972.
- Dixon CW. Smallpox. London: Churchill; 1962.
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. New York: Oxford University Press; 1991.
- Henderson DA. Smallpox eradication. Proc R Soc Lond B Biol Sci 1977;68:83-97.
- Arita I, Wickett J, Fenner F. Impact of population density on immunization programmes. J Hyg Camb
1986;96:459-66.
- Basu RN, Jezek Z, Ward NA. The eradication of smallpox from India. New Delhi: World Health Organization, South-east Asia Regional Office; 1979.
- Foege WH, Millar JD, Lane JM. Selective epidemiologic control in smallpox eradication. Am J Epidemiol 1971;94:311-5.
- Royal Commission on Vaccination. A report on vaccination and its results, based on evidence taken by the Royal Commission during the years 1889-1897. Vol 1. The text of the commission report. London: New Sydenham Society; 1898.
- Thomas DB, Arita I, McCormack WM, Khan MM, Islam S, Mack TM. Endemic smallpox in rural East Pakistan. II. Intravillage transmission and
infectiousness. Geneva: World Health Organization (WHO/SE/71.25); 1971.
- Thomas DB, McCormack WM, Arita I, Khan M, Islam S, Mack TM. Endemic smallpox in a rural area. Geneva: World Health Organization
(WHO/SE/69.11); 1969.
- Thomas DB, McCormack WM, Arita I, Khan MM, Islam S, Mack TM. Endemic smallpox in rural east Pakistan: I. Methodology, clinical and
epidemiological characteristics of cases, and intervillage transmission. Geneva: World Health Organization (WHO/SE/71.24); 1971.
- Arnt N, Morris L. Epidemiological characteristics of smallpox outbreaks in two small Brazilian villages. Geneva: World Health Organization
(WHO/SE/70.22); 1970.
- Pifer J, Adeoye CL. Characteristics of an epidemic of smallpox: Gerere hamlet, Nigeria, 1968. Geneva: World Health Organization (WHO/SE/68.5);
1968.
- Rao AR, Paramasivam TV, Kamalakshi S, Parasuraman AR, Shantha M. A short report of epidemiological investigations of smallpox outbreaks in
1969 in a few villages of Nellore district of Andrapradesh, India. Geneva; World Health Organization (WHO/SE/70.17); 1970.
- Rao AR. An outbreak of smallpox in Chingleput district, Madras. Geneva: World Health Organization (WHO/SE/68.6); 1968.
- Rangaraj AG. An outbreak of smallpox in a village in Afghanistan. Geneva: World Health Organization (WHO/SE/69.9); 1969.
- Heiner GG, Fatima N, McGrumb FR. A study of intrafamilial transmission of smallpox. Am J
Epidemiol, 1971;94:316-326.
- de Quadros CCA, Morris L, da Costa EA, Arnt N, Tigre CH. Epidemiology of variola minor in Brazil: A study of 33 outbreaks. Geneva: World Health
Organization (WHO/SE/71.32); 1971.
- de Costa EA, Morris L. Smallpox epidemic in a Brazilian community. Geneva: World Health Organization (WHO/SE/74.64); 1974.
- Rao AR. A short report on the epidemiological findings of smallpox outbreaks in the state of Tamil Nadu, July 1968--June 1969. Geneva: World
Health Organization (WHO/SE/70.19); 1970.
- Singh S. Some aspects of the epidemiology of smallpox in Nepal. Geneva: World Health Organization (WHO/SE/69.10); 1969.
- Rao AR. A short report on epidemiological findings of smallpox outbreaks in the city of Madras. Geneva: World Health Organization (WHO/SE/68.7);
1968.
- de Sario V. Field investigation of an outbreak of smallpox at Bawku, Ghana: May-October, 1967. Geneva: World Health Organization
(WHO/SE/69.24); 1969.
- Suleimanov GD, Mandokhel KK. Smallpox transmission on a bus. Geneva: World Health Organization (WHO/SE/72.41); 1972.
- Presthus GT, Sibiya JB. A persistent focus of smallpox in Botswana. Geneva: World Health Organization (WHO/SE/74.89); 1974.
- Litvinjenko S, Arsic B, Borjanovic S. Epidemiologic aspects of smallpox in Yugoslavia in 1972. Geneva: World Health Organization
(WHO/SE/73.57); 1973.
- Wehrle PF, Posch J, Richter KH, Henderson DA. An airborne outbreak of smallpox in a German hospital and its significance with respect to other
recent outbreaks in Europe. Bull World Health Organ 1970;43:669-79.
- Great Britain Ministry of Health. Smallpox, 1961-62. Reports on public health and medical subjects, No. 109. London: Her Majesty's Stationery
Office; 1963.
- Henderson DA, Yekpe M. Smallpox transmission in southern
Dahomey: a study of a village outbreak. Am J Epidemiol 1969;90:423-8.
- Smallpox Eradication Unit (WHO, Geneva). A smallpox outbreak in Merka town, Somalia. Geneva: World Health Organization (WHO/SE/78.123);
1978.
- Anderson RM, May RM. Vaccination against rubella and measles: quantitative investigations of different policies. J Hyg Camb 1983;90:259-325.
- Cliff AD, Haggett P. Statistical modeling of measles and influenza outbreaks. Stat Methods Med Res 1993;2:43-73.
- Henderson DA. Principles and lessons from the smallpox eradication
programme. Bull World Health Organ 1987;65:535-46.
1. The data in Table 2 indicate some age-specific risk, both among the vaccinated and unvaccinated. However, the risk does not appear to have a consistent pattern. For example, among
those with a history of vaccination living in a house with a smallpox patient, those >10 years of age had a higher attack rate than those <10 years of age. Yet, among the unvaccinated,
those <10 years of age had a higher attack rate than those >10 years of age. This relationship between vaccination status, age, and attack rate is repeated in the general population.
Selected Sources
- Anderson RM. Transmission dynamics and control of infectious disease agents. In: Anderson RM, May RM, editors. Population biology of infectious
diseases. Berlin: Springer-Verlag; 1982. p. 149-77.
- Anderson RM, May RM. Population biology of infectious diseases: Part I. Nature 1979;280:361-7.
- Anderson RM, May RM. Population biology of infectious diseases: Part II. Nature 1979;280:455-61.
- Anderson RM, May RM. Directly transmitted infectious diseases: control by vaccination. Science 1982;215:1053-60.
- Aron JL, May RM. The population dynamics of malaria. In: Anderson RM, editor. The population dynamics of infectious diseases: theory and
application. London: Chapman and Hall; 1982.
- Bartlett MS. Measles periodicity and community size. J Royal Stat Soc Series A 1957;120:48-60.
- Bardi J. Aftermath of a hypothetical smallpox disaster. Emerg Infect Dis 1999;5:547-51.
- Bartlett MS. Critical community size for measles in the United States. J Royal Stat Soc Series A 1960;123:37-44.
- Christie AR. Infectious diseases: epidemiology and clinical practice. 3rd ed. New York: Churchill Livingstone; 1980.
- Cliff AD, Haggett P. Statistical modeling of measles and influenza outbreaks. Stat Methods Med Res 1993;2:43-73.
- Deria A, Jezek Z, Foster S. Outbreak containment in the Somalia smallpox eradication programme. Geneva: World Health Organization
(WHO/SE/78.104); 1978.
- Frauenthal JC. Smallpox: When should routine vaccination be discontinued? The UMAP Expository Monograph Series. Boston: Birkhäuser; 1981.
- Glokpor GF, Agle AN. Epidemiological investigations--Smallpox Eradication Programme in Togo: 1969. Geneva: World Health Organization
(WHO/SE/70.21); 1970.
- Henderson DA. Smallpox: Clinical and epidemiological features. Emerg Infect Dis 1999;5:537-9.
- Henderson DA. The looming threat of
bioterrorism. Science 1999;283:1279-82.
- Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, Jahrling EP, et al.
Smallpox as a biological weapon: Medical and public health
management. JAMA 1999;281:2127-37.
- Kaufmann AF, Meltzer MI, Schmid GP. The economic impact of a bioterrorist attack: Are prevention and postattack intervention programs justifiable?
Emerg Infect Dis 1997;3:83-94.
- Mack TM. Smallpox in Europe, 1950-1971. J Infect Dis 1972;125:161-9.
- O'Toole T. Smallpox: An attack scenario. Emerg Infect Dis 1999;5:540-6.
- Pattanayak S, Sehgal PN, Raghavan NGS. Outbreaks of smallpox during 1968 in some villages of Jaipur district, Rajasthan. Geneva: World Health
Organization (WHO/SE/70.20); 1970.
- Smith ADM. Epidemiological patterns in directly transmitted human infections. In: Croll NA, Cross JH, editors. Human ecology and infectious diseases.
New York: Academic Press; 1983. p. 333-51.
- Statistical abstracts of the United States: 1999. 119th ed. Washington: U.S. Bureau of the
Census; 1999.
|
