Page Options
Media:
Contact Us
- 800–CDC–INFO
- (800-232-4636)
- 888–232–6348 (TTY)
- cdcinfo@cdc.gov
2007-08 U.S. INFLUENZA SEASON SUMMARY*
Synopsis:
During the 2007--2008 season, influenza activity** peaked in mid-February and was associated with greater overall mortality, and higher rates of hospitalizations among children aged 0-4 years compared with each of the previous three seasons. Influenza A (H1) viruses were most commonly isolated from October to mid-January, but influenza A (H3) viruses increased in circulation in January and predominated during the season overall. From late March through May, when overall influenza activity was declining, more influenza B than influenza A viruses were reported.
Laboratory Surveillance*:
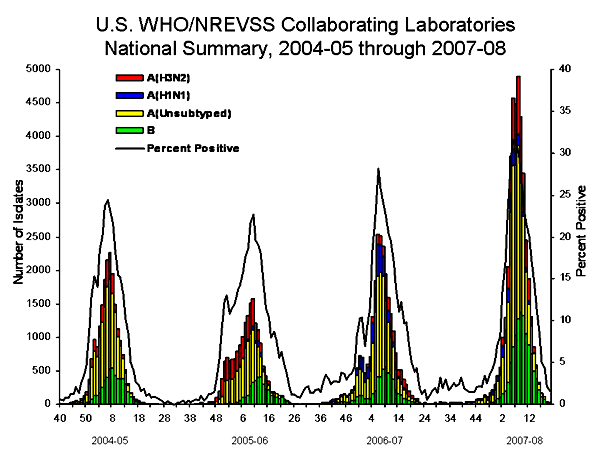
During September 30, 2007 – May 17, 2008, World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories in the United States tested 225,329 specimens for influenza viruses; 39,827 (18%) were positive. Of the positive specimens, 28,263 (71%) were influenza A viruses and 11,564 (29%) were influenza B viruses. Among the influenza A viruses, 8,290 (29%) were subtyped; 2,175 (26%) were influenza A (H1) and 6,115 (74%) were influenza A (H3) viruses. The proportion of specimens testing positive for influenza first exceeded 10% during the week ending January 12, 2008 (week 2), peaked at 32% during the week ending February 9, 2008 (week 6), and declined to < 10% during the week ending April 19, 2008 (week 16). The proportion was above 10% positive for 14 consecutive weeks. The peak percentage of specimens testing positive for influenza during the previous three seasons ranged from 22% to 34% and the peak occurred during mid-February to early March. During the previous three influenza seasons, the number of consecutive weeks during which more than 10% of specimens tested positive for influenza ranged from 13 to 17 weeks.
During the 2007--08 influenza season, more influenza A viruses than influenza B viruses were identified in all nine surveillance regions¥¥ however, the predominant influenza A virus varied by region. Influenza A (H1) was most commonly reported in two of the nine surveillance regions (Mountain and Pacific), and influenza A (H3) was most commonly reported in the remaining seven surveillance regions (East North Central, East South Central, Mid-Atlantic, New England, South Atlantic, West North Central, and West South Central). Although influenza A viruses predominated in all nine regions a larger proportion of the influenza viruses detected were influenza B in the New England and Mid Atlantic regions where 45% and 44% of viruses reported were influenza B viruses. However, less influenza B activity was seen in the East North Central, East South Central, and South Atlantic regions. The timing of peak activity varied slightly across the nine surveillance regions, with activity in all regions peaking between late January and late February.
View Full Screen | View Chart Data
Antigenic Characterization:
Between September 30, 2007 and June 19, 2008 , CDC has antigenically characterized 1,161 influenza viruses collected by U.S. laboratories: 407 influenza A (H1N1) viruses, 404 influenza A (H3N2) viruses, and 350 influenza B viruses. Of the 407 influenza A (H1N1) viruses, 270 (66%) were characterized as antigenically similar to A/Solomon Islands/3/2006, the influenza A (H1N1) component of the 2007--08 Northern Hemisphere influenza vaccine. One hundred sixteen (29%) viruses were characterized as A/Brisbane/59/2007-like. Of the 404 influenza A (H3N2) viruses, 91 (23%) were characterized as similar to A/Wisconsin/67/2005, the influenza A (H3) component of the 2007--08 Northern Hemisphere influenza vaccine. Two hundred forty-three (60%) viruses were characterized as A/Brisbane/10/2007-like.
Influenza B viruses currently circulating can be divided into two antigenically distinct lineages represented by B/Victoria/02/87 and B/Yamagata/16/88 viruses. Of the 350 influenza B viruses characterized, 342 (98%) were identified as belonging to the B/Yamagata lineage, and 304 (89%) of these viruses were similar to B/Florida/4/2006. The remaining eight (2%) of the 350 influenza B viruses characterized belong to the B/Victoria lineage; and of these, six (75%) were similar to B/Ohio/01/2005, an antigenic equivalent to B/Malaysia/2506/2004, the influenza B component for the 2007--08 Northern Hemisphere influenza vaccine.
Composition of the 2008-09 Influenza Vaccine:
The Food and Drug Administration's Vaccines and Related Biological Products Advisory Committee recommended that the 2008--09 trivalent influenza vaccine for the United States contain A/Brisbane/59/2007-like (H1N1), A/Brisbane/10/2007-like (H3N2), and B/Florida/4/2006-like viruses. This represents a change in all three components from the 2007--08 influenza vaccine formulation used in the United States. These recommendations were based on antigenic analyses of recently isolated influenza viruses, epidemiologic data, post-vaccination serologic studies in humans, and the availability of candidate vaccine strains and reagents.
Resistance to Antiviral Medications:
In the United States, two classes of antiviral drugs are approved by the Food and Drug Administration for use in treating or preventing influenza virus infections: neuraminidase inhibitors (oseltamivir and zanamivir) and adamantanes (amantadine and rimantidine). During the 2007--08 influenza season, a small increase in the number of influenza viruses resistant to the neuraminidase inhibitor oseltamivir was observed. All of the oseltamivir-resistant viruses were influenza A (H1N1) isolates that shared a single genetic mutation (H274Y, N2 neuraminidase molecule numbering) that confers oseltamivir resistance. Among specimens collected from October 1, 2007 to May 17, 2008, 111 (10.9%) of the 1,020 influenza A (H1N1) viruses tested were found to be resistant to oseltamivir, an increase from four (0.7%) of 588 influenza A (H1N1) viruses tested during the 2006--07 season. No resistance to oseltamivir was identified among the 444 influenza A (H3N2) or 305 influenza B viruses tested. It is estimated based on the overall proportion of H1N1 viruses among all influenza viruses reported during the 2007-08 season that 2.0% of all viruses were resistant to oseltamivir. All tested viruses were sensitive to zanamivir.
Adamantane resistance continues to be high among influenza A (H3N2) viruses with 524 (99.8%) of 525 influenza A (H3N2) viruses tested being resistant to the adamantanes. Adamantane resistance among influenza A (H1N1) viruses has been detected at a lower level. Of the 918 influenza A (H1N1) viruses tested, 98 (10.7%) were resistant to the adamantanes. None of the oseltamivir-resistant influenza A (H1N1) viruses identified during the 2007--08 season were resistant to adamantanes.
CDC continues to recommend the use of oseltamivir and zanamivir for the treatment and prevention of influenza based on the level of oseltamivir resistance observed only in one influenza subtype, influenza A (H1N1), persisting high levels of resistance to adamantanes in influenza A (H3N2) viruses, and the predominance of influenza A (H3N2) viruses circulating in the United States during the 2007-08 season with co-circulation of influenza B viruses. Use of amantadine or rimantidine is not recommended. Guidance on influenza antiviral use can be found at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5606a1.htm.
Pneumonia and Influenza (P&I) Mortality Surveillance*:
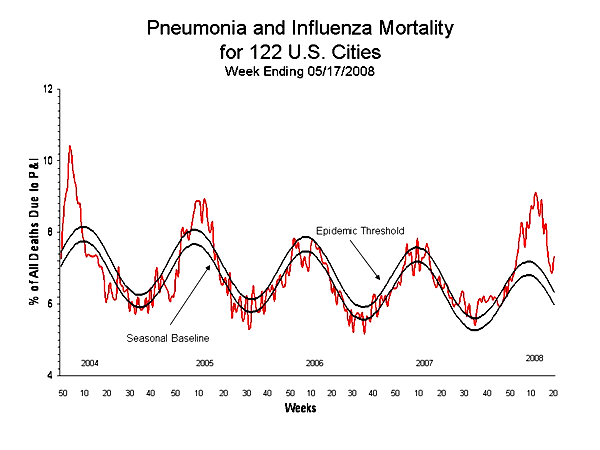
During the 2007--08 influenza season, the percentage of deaths attributed to pneumonia and influenza (P&I) exceeded the epidemic threshold†† for 8 consecutive weeks in the 122 Cities Mortality Reporting System during the weeks ending January 12--May 17, 2008 (weeks 9--16). The percentage of P&I deaths peaked at 9.1% during the week ending March 15, 2008 (week 11). During the previous three influenza seasons, the peak percentage of P&I deaths has ranged from 7.7% to 8.9% and the total number of weeks the P&I ratio exceeded the epidemic threshold has ranged from one to 11. The P&I baseline and epidemic threshold values are projected for each season at the onset of that season and are based on data from the previous five years. The robust regression model used to calculate the 122 Cities Mortality Reporting System baseline and epidemic threshold values was recently modified. This new methodology better takes into account shifts in the long term trends of the 122 Cities data, and will be used in the upcoming 2008-09 influenza season to project the baseline and epidemic threshold values.
View Full Screen
Influenza-Associated Pediatric Mortality*:
As of June 19, 2008, 83 deaths associated with laboratory-confirmed influenza infections have occurred among children aged < 18 years during the 2007--08 influenza season that were reported to CDC. These deaths were reported from 33 states (Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Florida, Georgia, Illinois, Indiana, Iowa, Maine, Maryland, Massachusetts, Michigan, Minnesota, Mississippi, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Tennessee, Texas, Utah, Vermont, Washington, and Wisconsin). Among the 83 cases, the mean and median age was 6.4 years and 5.0 years, respectively; seven children were aged < 6 months, 16 were aged 6--23 months, 18 were aged 2--4 years, and 42 were aged 5--17 years. Of the 79 cases for which the influenza virus type was known, 51 were influenza A viruses, 27 were influenza B viruses, and one had co-infection with influenza A and B viruses. Of the 63 cases aged 6 months and older for whom vaccination status was known, 58 (92%) had not been vaccinated against influenza according to the 2007 Advisory Committee on Immunization Practices recommendations. These data are provisional and subject to change as more information becomes available.
Influenza-Associated Pediatric Hospitalizations*:
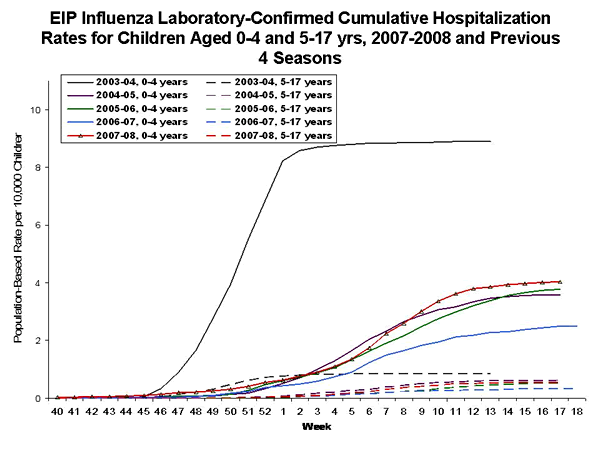
Pediatric hospitalizations associated with laboratory-confirmed influenza infections are monitored in two population-based surveillance networks: the Emerging Infections Program (EIP) and the New Vaccine Surveillance Network (NVSN).† During September 30, 2007--May 3, 2008, the preliminary influenza-associated hospitalization rate reported by EIP for children aged 0-17 years was 1.53 per 10,000. For children aged 0--4 years and 5--17 years, the rate was 4.03 per 10,000 and 0.55 per 10,000, respectively.
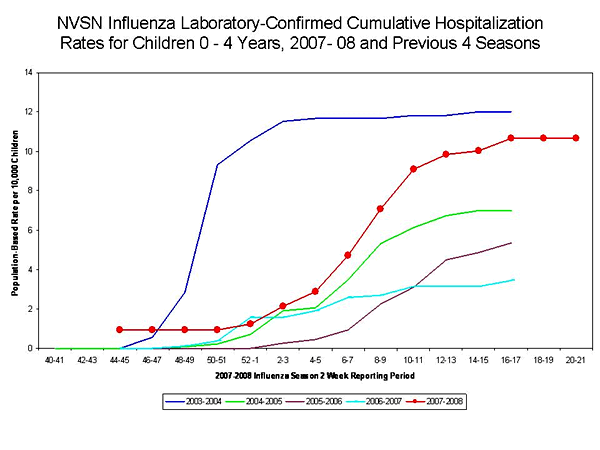
During November 4, 2007--May 3, 2008, the preliminary laboratory-confirmed influenza-associated hospitalization rate for children aged 0--4 years in NVSN was 10.66 per 10,000. Rate estimates are preliminary and are subject to change as data are finalized.
View Full Screen
TThe end-of-season hospitalization rate for NVSN in the previous four seasons ranged from 3.5 (2006--07) to 12.0 (2003--04) per 10,000 children aged 0-4 years. The end-of-season hospitalization rate for EIP in the previous four seasons ranged from 2.5 (2006--07) to 8.9 (2003--04) per 10,000 children aged 0-4 years, and 0.3 (2006--07) to 0.8 per (2003--04) per 10,000 children aged 5--17 years. Differences in rate estimates between the NVSN and EIP systems are likely the result of different case-finding methods, diagnostic tests used, and the populations monitored.
View Full Screen
Outpatient Illness Surveillance:*
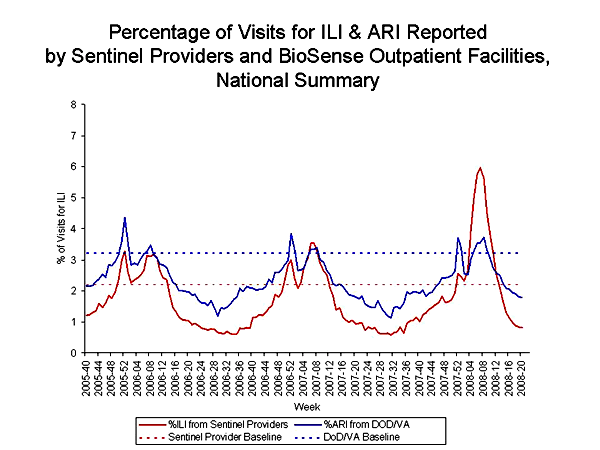
The weekly percentage of patient visits to U.S. sentinel providers for influenza-like illness (ILI)§ met or exceeded national baseline levels (2.2%)§§ during the weeks ending December 29, 2007--March 22, 2008 (weeks 52--12) and peaked at 6.0% for the week ending February 16, 2008 (week 7). During the previous three influenza seasons, the peak percentage of patient visits for ILI ranged from 3.2% to 5.4% and occurred during mid-February to early March. Regional timing of peak ILI activity mirrored what was seen in the virologic data. Activity in all nine regions peaked in early to late February. The peak percentage of visits for ILI in the nine surveillance regions ranged from 3.9% to 10.8%. The number of weeks at or above region specific baselines ranged from four weeks in the Mid Atlantic region to 14 weeks in the East North Central region, with the other seven regions reporting between 10 and 13 weeks at or above baseline. ILI was at or above the national baseline of 2.2% for 13 weeks.
The weekly percentage of visits to Department of Veteran’s Affairs (VA) and Department of Defense (DoD) BioSense¶ outpatient clinics for acute respiratory illness (ARI)¶¶ was at or above national baseline levels¶¶¶ during the weeks ending December 29, 2007--January 5, 2008 (weeks 52--1), and February 2--March 1, 2008 (weeks 5--9). Outpatient clinic visits for ARI peaked twice, once at 3.7% during the week ending December 29, 2007 (week 52), and again at 3.7% for the week ending February 23, 2008 (week 8). During the previous three influenza seasons, the peak percentage of patient visits for ARI has ranged from 3.4% to 4.5% and occurred during mid to late February. The peak percentage of visits for ARI on a regional level ranged from 1.9% in the Mid-Atlantic region to 4.6% in West South Central region. The number of weeks at or above region specific baselines ranged from one in the New England region to seven in the West North Central region with the other seven regions reporting between two and four weeks at or above their region-specific baselines.
The increase in the percentage of visits for ILI and ARI during the week ending December 29, 2007 (week 52) might have been influenced by a reduction in routine health-care visits during the holiday season, as has occurred during previous seasons.
View Full Screen | View Chart Data
| View Full Screen Or click on each region graphic, to view larger images |

|
State-Specific Activity Levels:*
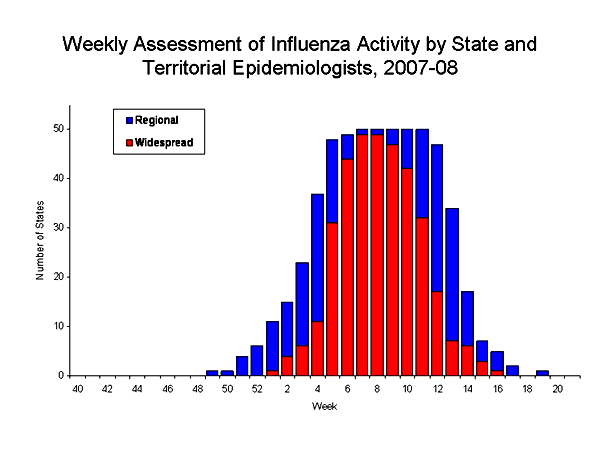
State and territorial epidemiologists report the geographic distribution of influenza in their state through a weekly influenza activity code.*** The geographic distribution of influenza activity peaked during the weeks ending February 16 and February 23, 2008 (weeks 7 and 8), when 49 states reported widespread activity and one state reported regional activity. All 50 states reported widespread influenza activity for at least two weeks during the 2007-08 season. No state reported widespread influenza activity during the weeks ending April 26--May 17, 2008 (weeks 17--20). The peak number of states reporting widespread or regional activity during the previous three seasons has ranged from 41 to 48 states.
View Full Screen
For additional information on the 2007-2008 influenza season please see the season summary MMWR (Update: Influenza Activity --- United States and Worldwide, 2007--08 Season. MMWR Morb Mortal Wkly Rep. 2008 June 27;57(25):692-700).
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5725a5.htm
--------------------------------------------------------------------------------
- Page last updated: October 6, 2008
- Content Source: Coordinating Center for Infectious Diseases (CCID)
- National Center for Immunization and Respiratory Diseases (NCIRD)

