ISSN: 1080-6059
Volume 15, Number 1–January 2009
Research
Human Infection with Highly Pathogenic Avian Influenza Virus (H5N1) in Northern Vietnam, 2004–2005
Nguyen Duc Hien, Nguyen Hong Ha, Nguyen Tuong Van, Nguyen
Thi Minh Ha, Trinh Thi Minh Lien, Nguyen Quoc Thai, Van Dinh Trang, Takuro
Shimbo ![]() , Yoshimitsu Takahashi, Yasuyuki Kato, Akihiko Kawana, Samu Akita, and Koichiro Kudo
, Yoshimitsu Takahashi, Yasuyuki Kato, Akihiko Kawana, Samu Akita, and Koichiro Kudo
Author affiliations: National Institute of Infectious and
Tropical Diseases, Hanoi, Vietnam (N.D. Hien, N.H. Ha, N.T. Van, N.T.M. Ha, T.T.M.
Lien, N.Q. Thai, V.D. Trang); and International Medical Center of Japan, Tokyo,
Japan (T. Shimbo, Y. Takahashi, Y. Kato, A. Kawana, S. Akita, K. Kudo)
Suggested citation for this article
Abstract
We performed a retrospective case-series study of patients
with influenza A (H5N1) admitted to the National Institute of Infectious and
Tropical Diseases in Hanoi, Vietnam, from January 2004 through July 2005 with
symptoms of acute respiratory tract infection, a history of high-risk exposure
or chest radiographic findings such as pneumonia, and positive findings for
A/H5 viral RNA by reverse transcription–PCR. We investigated data from 29
patients (mean age 35.1 years) of whom 7 (24.1%) had died. Mortality rates were
20% (5/25) and 50% (2/4) among patients treated with or without oseltamivir (p
= 0.24), respectively, and were 33.3% (5/15) and 14.2% (2/14) among patients
treated with and without methylprednisolone (p = 0.39), respectively. After exact
logistic regression analysis was adjusted for variation in severity, no significant
effectiveness for survival was observed among patients treated with oseltamivir
or methylprednisolone.
Human infection with the highly pathogenic avian influenza A virus (H5N1) was discovered in Hong Kong Special Administrative Region, People's Republic of China, in 1997 (1–3). It has since been identified in other countries, primarily in Southeast Asia. Among 100 confirmed infected patients, 46 have died in Vietnam since 2003 (4,5).
Severe viral pneumonia accompanied by diffuse alveolar damage develops in patients infected with influenza virus (H5N1) (6). High viral load causes intense cytokine reactions and inflammation (7). Clinical factors that might be associated with severity include age, delayed consultation, lower respiratory tract lesions, and leukopenia (4,8–10). However, few cases have reported which factors, including patient management, affect outcomes. Our study reviews the clinical courses of patients treated in Hanoi, Vietnam, and investigates the association between clinical findings and survival.
The effects of oseltamivir and other neuraminidase inhibitors have been demonstrated in experimental models (11–13), but their outcomes in humans have not been verified. Randomized controlled trials would be optimal for investigating the effectiveness of oseltamivir compared with placebo, but they are not an option because of ethical issues. Therefore, this issue can only be addressed through observational studies. Despite limited empirical evidence, the World Health Organization (WHO) reported that oseltamivir improved survival (14) and recommended treatment with oseltamivir because of high mortality rates associated with influenza A virus (14,15). Patients from northern Vietnam are described in detail.
Methods
We investigated patients infected with influenza A virus (H5N1) who were referred to the National Institute of Infectious and Tropical Diseases in Hanoi, Vietnam, from other local hospitals from January 2004 through July 2005. Pediatric patients were admitted to another institution in Hanoi and were excluded from the present study. A WHO inspection team at the National Institute for Hygiene and Epidemiology in Hanoi virologically confirmed H5N1 subtype infection in the patients by using a reverse transcription–PCR (RT-PCR) for influenza A/H5. We investigated only patients with H5N1 subtype infection determined from symptoms of acute respiratory tract infection, a history of high-risk exposure, or chest radiographic findings such as pneumonia. All patients were reported to WHO as having confirmed infection with avian influenza virus (H5N1). We excluded other patients with positive RT-PCR results because of reasons described below. The study was reviewed and approved by the ethics committees at the International Medical Center of Japan and the National Institute of Infectious and Tropical Diseases in Vietnam.
Data were obtained for general characteristics, history of high-risk exposure, medical history, symptoms, signs, microbiologic and biochemical test results, chest radiographic findings, treatment strategies, and outcomes from medical records from April through October 2006.
We investigated associations between clinical findings and survival by using univariate analysis. Initial laboratory and chest radiographic findings after hospitalization were recorded in the medical charts and used. The relationship between survival and treatment with oseltamivir or methylprednisolone was investigated by adjusting for factors related to severity in an exact logistic regression analysis, which is appropriate for small amounts or unbalanced binary data (16). Because the study cohort was small, deaths were few and overfitting was possible (17,18); only 1 covariate could be added for adjustment into the logistic regression model. Therefore, we used leukocyte counts, platelet counts, aspartate aminotransferase (AST) levels, and urea nitrogen levels as an adjustment for severity because these values are associated with reported outcomes (14). Also, many missing observations prevented adjustment using albumin levels. Data were analyzed by using the Wilcoxon test, χ2 test, and Fisher exact test when appropriate and the statistical package SAS version 8.2 (SAS Institute, Cary, NC, USA).
Results
Among 41 patients who were hospitalized from January 2004 through July 2005 and had positive RT-PCR results, 12 were excluded from the study (3 patients whose medical records were unavailable; 2 patients related to persons with confirmed H5N1 subtype pneumonia who were asymptomatic, positive for viral RNA, and treated with prophylactic oseltamivir; and 7 patients who had some illnesses, particularly respiratory diseases, which complicated interpretation of the clinical course or chest radiographic findings). We therefore studied 29 patients with clinically and virologically confirmed influenza A (H5N1) infection.
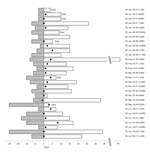 |
Figure. Clinical course of 29 patients infected with highly pathogenic avian influenza virus (H5N1), northern Vietnam, 2004–2005... |
Table 1 shows the general characteristics of the patients, and the Figure shows the clinical course from onset of disease to hospitalization and discharge. Patients ranged in age from 14 to 67 years and with a mean age of 35.1 years. A total of 25 patients were given 150 mg/day of oseltamivir, and 15 were treated with methylprednisolone (initial dose 40–160 mg/day, median dose 80 mg/day). Seven (24.1%) of the 29 patients died. No significant associations were found between mortality rates and age (p = 0.57), sex (p = 0.68), history of high-risk exposure (contact with poultry [p = 1.00], contact with sick poultry [p = 1.00], and contact with sick poultry or persons [p = 1.00]). Three of 6 patients from a family infected with H5N1 subtype died, and 4 of 23 patients without such an association died (p = 0.13). Duration between onset of disease and hospitalization was not associated with higher mortality rates (p = 0.98).
Table 2 shows initial laboratory findings at hospitalization. Leukopenia (neutropenia), thrombocytopenia, hypoalbuminemia, and increased AST and urea nitrogen levels were associated with increased deaths.
Five (20.0%) of the 25 patients treated with oseltamivir died, as did 2 (50.0%) of 4 who were not treated (odds ratio 0.25, 95% confidence interval [CI] 0.03–2.24, p = 0.24). To adjust for variation in disease severity among patients, exact logistic regression was performed by using leukocyte counts, platelet counts, AST levels, and urea nitrogen levels. Adjusted odds ratios for deaths among patients treated with oseltamivir were 0.15 (95% CI 0.00–2.57, p = 0.19), 0.16 (95% CI 0.00–2.23, p = 0.17), 0.54 (95% CI 0.02–11.85, p = 1.00), and 0.28 (95% CI 0.01–5.16, p = 0.55), respectively, for the 4 adjustments for disease severity.
The time between the onset of symptoms and initiation of treatment with oseltamivir varied (Table 1, Figure). The mortality rates were 20% (3/15) and 20% (2/10) when treatment with oseltamivir was started within and after 7 days of disease onset.
Methylprednisolone was given to 15 of 29 patients. Five (33.3%) of these 15 patients died, and 2 (14.3%) of 14 patients who were not given this drug died (odds ratio 3.0, 95% CI 0.48–18.93, p = 0.39). Exact logistic regression after adjustment for severity by using leukocyte counts, platelet counts, AST levels, or urea nitrogen levels showed odds ratios for deaths among patients treated with methylprednisolone of 0.74 (95% CI 0.00–9.57, p = 0.82), 1.82 (95% CI 0.18–25.48, p = 0.89), 1.14 (95% CI 0.07–18.92, p = 1.00), and 2.43 (95% CI 0.28–31.69, p = 0.61), respectively.
Thirteen patients were treated with oseltamivir and methylprednisolone. The regression model that included these 2 drugs and interactions did not show effectiveness of either drug.
Discussion
The overall mortality rate of 24.1% in this study was lower than rates in previous studies and WHO reports. Table 3 summarizes the characteristics of patients from previous studies. Patients in the present study were older because pediatric patients were excluded because of treatment elsewhere. WHO has reported that the mortality rate of 73% for infection with H5NI subtype is highest in persons 10–19 years of age, and that patients 20–39 years of age account for >60% of the deaths (22). The expected mortality rate would be 51.8% if our case-patients had the same age-specific mortality rate as in a WHO report (14). The lower mortality rate in our study could not be explained by an age difference. The relatively high leukocyte count and factors related to outcomes suggest that a reasonably large number of mildly infected patients might have been included, although chest radiographs showed variable progression in lesions from mild to severe.
Persons who died were concentrated in the early period of the study, especially in 2004. Virus genotype and load data could provide useful information on pathogenesis and outcome. However, these data were not available.
Factors affecting outcome were leukocyte and platelet counts, and albumin, ALT, and urea nitrogen levels. Results were consistent with previous findings (14) and suggested that outcome is related to lesions in several organs.
Although the mortality rate was lower among patients treated with oseltamivir, differences were not significant. Exact logistic regression after adjustments for laboratory results yielded an odds ratio of 0.15–0.54 for death. The small number of patients prevented valid adjustment, and confounding factors might not have been sufficiently eliminated. A larger patient cohort should be able to adjust for severity of disease.
If one considers the possibility of confounding factors, the reason oseltamivir was not prescribed should be investigated. If oseltamivir was withheld from patients with severe infections and administered only to those with milder symptoms, the drug would apparently be more effective. Among 4 patients who were not prescribed oseltamivir, initial RT-PCR results were negative for 1 patient, who subsequently died. Oseltamivir was unavailable for treatment of another patient who died. The other 2 surviving patients were not prescribed oseltamivir because their chest radiographs showed only minimal lesions. Therefore, withholding oseltamivir was not associated with disease severity.
Higher doses of oseltamivir or longer drug administration have improved outcomes in animal models (23,24). Because all patients in our study were given oseltamivir at a dose of 150 mg/day, we could not investigate the effect of a higher dose.
Mortality rates were higher in patients treated with methylprednisolone than in those not treated with this drug. This finding can be explained by disease severity because severely ill patients were more likely to be given methylprednisolone. However, even after we adjusted for this confounding effect, no beneficial effect of methylprednisolone was observed. Further, an experimental model has recently raised doubt about the effect of cytokine suppression (25).
Our study described patients infected with influenza A virus (H5N1) in Hanoi, Vietnam. These patients had lower mortality rates than those reported in other studies. The reason for the low mortality rate could not be investigated thoroughly without virologic information. Oseltamivir was prescribed in 25 of 29 patients, and their mortality rate was apparently decreased, although the patient cohort was too small to generate sufficient statistical power. In addition, since our study was an observational study, these findings might have been influenced by confounding factors. Further detailed observations from a larger number of patients are required.
Acknowledgments
We thank the staffs of the National Institute of Infectious and Tropical Diseases, Bach Mai Hospital, and the National Institute of Hygiene and Epidemiology for diagnosing the infections and treating patients; and Nguyen Thi Le Hang, Pham Thi Phuong Thuy, and Toshie Manabe for invaluable help in coordinating this study.
This study was supported by a research grant (Research on Emerging and Re-emerging Infectious Diseases) from the Ministry of Health, Labour and Welfare, Japan, to K.K.
Dr Hien is the director general at the National Institute of Infectious and Tropical Diseases in Hanoi, Vietnam. His research interests are clinical practice and research of infectious and tropical diseases.
References
- Centers for Disease Control and Prevention. Isolation of avian influenza A(H5N1) viruses from humans—Hong Kong, May–December 1997. JAMA. 1998;279:263–4. PubMed DOI
- Centers for Disease Control and Prevention. Update: isolation of avian influenza A(H5N1) viruses from humans—Hong Kong, 1997–1998. JAMA. 1998;279:347–8. PubMed DOI
- Ku AS, Chan LT. The first case of H5N1 avian influenza infection in a human with complications of adult respiratory distress syndrome and Reye's syndrome. J Paediatr Child Health. 1999;35:207–9.
- Tran TH, Nguyen TL, Nguyen TD, Luong TS, Pham PM, Nguyen VC, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350:1179–88. PubMed DOI
- World Health Organization. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO, 2007. Geneva: The Organization [cited 2008 Oct 2]. Available from http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_09_10/en/index.html
- To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–6. PubMed DOI
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–7. PubMed DOI
- Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–71. PubMed DOI
- Chotpitayasunondh T, Ungchusak K, Hanshaoworakul W, Chunsuthiwat S, Sawanpanyalert P, Kijphati R, et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005;11:201–9.
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Long MD, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–85. PubMed DOI
- Gubareva LV, McCullers JA, Bethell RC, Webster RG. Characterization of influenza A/HongKong/156/97 (H5N1) virus in a mouse model and protective effect of zanamivir on H5N1 infection in mice. J Infect Dis. 1998;178:1592–6. PubMed DOI
- Leneva IA, Roberts N, Govorkova EA, Goloubeva OG, Webster RG. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 2000;48:101–15. PubMed DOI
- Leneva IA, Goloubeva O, Fenton RJ, Tisdale M, Webster RG. Efficacy of zanamivir against avian influenza A viruses that possess genes encoding H5N1 internal proteins and are pathogenic in mammals. Antimicrob Agents Chemother. 2001;45:1216–24. PubMed DOI
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358:261–73. PubMed DOI
- Schunemann HJ, Hill SR, Kakad M, Bellamy R, Uyeki TM, Hayden FG, et al. WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus. Lancet Infect Dis. 2007;7:21–31. PubMed DOI
- Mehta CR, Patel NR. Exact logistic regression: theory and examples. Stat Med. 1995;14:2143–60. PubMed DOI
- Concato J, Feinstein AR, Holford TR. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118:201–10.
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. PubMed DOI
- Oner AF, Arslan S, Akdeniz H, Sahin HA, Cesur Y, Epcacan S, et al. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N Engl J Med. 2006;355:2179–85. PubMed DOI
- Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen, Hadisoedarsuno W, Purba W, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355:2186–94. PubMed DOI
- Buchy P, Mardy S, Vong S, Toyoda T, Aubin JT, Miller M, et al. Influenza A/H5N1 virus infection in humans in Cambodia. J Clin Virol. 2007;39:164–8. PubMed DOI
- World Health Organization. Epidemiology of WHO-confirmed human cases of avian influenza A(H5N1) infection. Wkly Epidemiol Rec. 2006;81:249–60.
- Govorkova EA, Rehg JE, Krauss S, Yen HL, Guan Y, Peiris M, et al. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J Virol. 2005;79:2191–8. PubMed DOI
- Yen HL, Monto AS, Webster RG, Govorkova EA. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J Infect Dis. 2005;192:665–72. PubMed DOI
- Salomon R, Hoffmann E, Webster RG. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A. 2007;104:12479–81. PubMed DOI
Figure
Tables
Table 1. Characteristics of 29
patients infected with highly pathogenic avian influenza virus (H5N1),
northern Vietnam, 2004–2005
Table 2. Initial laboratory and chest radiographic results
for 29 patients infected with highly pathogenic avian influenza virus (H5N1),
northern Vietnam, 2004–2005
Table 3. Comparison of studies of influenza A virus (H5N1)–infected patients
Suggested Citation for this Article
Hien ND, Ha NH, Van NT, Ha NTM, Lien TTM, Thai NQ, et al. Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004–2005. Emerg Infect Dis [serial on the Internet]. 2009 Jan [date cited]. Available from http://www.cdc.gov/EID/content/15/1/19.htm
DOI: 10.3201/eid1501.080073
Please use the form below to submit correspondence to the authors or contact them at the following address:
Takuro Shimbo, Research Institute, International Medical Center of Japan, 1-21-1 Toyama, Shinjuku, Tokyo 162-8655, Japan; email: tshimbo@ri.imcj.go.jp
Please contact the EID Editors at eideditor@cdc.gov
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
This page posted December 23, 2008
Centers for Disease Control and Prevention, 1600 Clifton Rd, Atlanta, GA 30333, U.S.A
Tel: (404) 639-3311 / Public Inquiries: (404) 639-3534 / (800) 311-3435