Pandemic Planning Update IV
Documents in PDF format require the Adobe Acrobat Reader®. If you experience problems with PDF documents, please download the latest version of the Reader®.
Contents:
Report from Secretary Michael O. Leavitt
U.S. Department of Health and Human Services
July 18, 2007
“Because of its unique nature, responsibility for preparedness and response to a pandemic extends across all levels of government and all segments of society. No single entity alone can prevent or mitigate the impact of a pandemic.” -- National Strategy for Pandemic Influenza, November 2005
Sisseton, South Dakota, (population 2,572) is actively preparing to survive an influenza pandemic, should that or any other public health emergency arise. Local officials, working closely with tribal leaders from the Sisseton-Wahpeton Oyate Native American community, have helped guide residents on how to set aside a two-week food supply. They’ve launched a campaign to encourage seasonal influenza vaccinations. They’ve even planned how to redirect traffic to and from the local high school, their designated site for any needed mass vaccinations.
“In the past few months, the media buzz around ‘bird flu’ has died down, but the H5N1 strain of avian influenza has not. It remains a serious danger that we must all face together.”
--Secretary Mike Leavitt, HHS
May 15, 2007
Over the past year State, local, tribal, and territorial governments along with people from all walks of life began to share responsibility for pandemic planning. Thanks to thousands of dedicated political and health care leaders, employers, school leaders, the media, individuals, and families, we can now mobilize a more effective response to a pandemic than we could a year ago.
 Our framework continues to be the President’s three strategic pillars—preparedness and communication, surveillance and detection, and response and containment. Since 2005, Congress has supported these efforts, supplying $5.6billion in funding to the U.S. Department of Health and Human Services (HHS) to help mobilize the nation.
Our framework continues to be the President’s three strategic pillars—preparedness and communication, surveillance and detection, and response and containment. Since 2005, Congress has supported these efforts, supplying $5.6billion in funding to the U.S. Department of Health and Human Services (HHS) to help mobilize the nation.
Driving our sense of urgency is a two-part reality: (1) that six months are needed from the start of a pandemic before a well-matched vaccine can be developed and (2) that, even then, current domestic manufacturing capacity is insufficient to provide a pandemic vaccine for all Americans in advance of a possible (many experts would say “likely”) second pandemic wave.
HHS confronted this reality and committed to addressing it. Early promising results of that commitment are beginning to appear on the horizon.
For the first time in our history, we have licensed a pre-pandemic vaccine that, if combined successfully with dose-sparing additives called adjuvants and manufactured in large quantities, may help carry us through the early months of a pandemic. Furthermore, vaccine and related industries are accelerating the development of technologies to move vaccines more quickly through the production pipeline and make them more effective.
Complementing our investments in vaccines are our investments in antiviral drugs. Enough antiviral drugs are now stockpiled among State and Federal governments for the treatment of more than 50 million Americans affected by pandemic influenza. Research proceeds on a third antiviral medication that can be injected directly into the body, so that those too ill to swallow the capsule form of current antiviral drugs can use this new option.
 We are tapping into the power of the mass media to help bring the pandemic preparedness message to every American. We held six exercises with key media leaders and senior government officials to test the validity of our own planning assumptions. We launched a series of television and radio public service announcements in English and Spanish to raise awareness about pandemic influenza planning. We convened a forum for leaders from all walks of life to begin a dynamic dialogue on preparing for a pandemic. And we launched our first “blog summit,” a five-week event joining leaders and the public in an open online conversation to help shape thinking about preparedness across society.
We are tapping into the power of the mass media to help bring the pandemic preparedness message to every American. We held six exercises with key media leaders and senior government officials to test the validity of our own planning assumptions. We launched a series of television and radio public service announcements in English and Spanish to raise awareness about pandemic influenza planning. We convened a forum for leaders from all walks of life to begin a dynamic dialogue on preparing for a pandemic. And we launched our first “blog summit,” a five-week event joining leaders and the public in an open online conversation to help shape thinking about preparedness across society.
Around the country, governments at every level are taking up their share of pandemic planning responsibilities. Over the past 18 months, States, territories, and tribes held 59 planning summits to identify what decisions need to be made and who needs to make them—before, during, and after a pandemic. The National Governors’ Association has begun holding multistate, regional summits so States can decide how to rely on each other’s strengths in a public health crisis.
 We also help support local decision-making processes by bringing the best minds together nationally and developing guidelines so that communities can decide on local courses of action.
We also help support local decision-making processes by bringing the best minds together nationally and developing guidelines so that communities can decide on local courses of action.
Communities now have a series of interim guidelines to coach them through their planning. The centerpiece is the Centers for Disease Control and Prevention’s (CDC’s) Community Strategy for Pandemic Influenza Mitigation. It introduces the Pandemic Severity Index, which is much like the National Weather Service’s hurricane intensity scale.
At a (low) level 2 pandemic, for example, a community can encourage people to stay home if they become ill. In a more severe level 4 or 5 pandemic, a community would help slow the spread of the virus by quickly adding multiple actions—such as closing schools, keeping children at home, promoting social distancing in the workplace—that would help maintain the community’s social and economic stability.
Interim advice is also now available on using surgical masks and respirators. Little evidence now exists about the benefits of masks and respirators in preventing the spread of influenza. However, while we gather evidence, this interim guidance can help local communities begin to consider the issue.
 In the first six months of a pandemic, access to pre-pandemic vaccines may be limited—depending upon the progress in the use of adjuvants and the enhancements of manufacturing capacity. Who would receive those few vaccines? This decision would involve everyone with a stake in the outcome—that is, the American public. Several public discussions of vaccine prioritization have already been held, with input from ethicists, elected and public health officials, and people in communities around the country. This dialogue will continue and lead to guidance in the near future.
In the first six months of a pandemic, access to pre-pandemic vaccines may be limited—depending upon the progress in the use of adjuvants and the enhancements of manufacturing capacity. Who would receive those few vaccines? This decision would involve everyone with a stake in the outcome—that is, the American public. Several public discussions of vaccine prioritization have already been held, with input from ethicists, elected and public health officials, and people in communities around the country. This dialogue will continue and lead to guidance in the near future.
HHS has also made available two key test exercises for State and local government leaders to role-play their way through decisions about closing schools and carrying out mass vaccinations. The CDC provided communities with a “pre-game” structure and “post-game” analysis, so each community can practice and improve its ability to take care of itself in a crisis.
As we closely watch the world’s avian influenza hotspots, we also coordinate with other government agencies and global partners as well as affected and at-risk countries to monitor developments and offer assistance. And we continue to call on countries everywhere to support the World Health Organization’s (WHO’s) May 2007 resolution to allow the fair and transparent sharing of influenza specimens and viruses through the Global Influenza Surveillance Network.
But much remains to be done. Each day we improve our ability to respond to this emerging public health challenge, so that we will be better prepared tomorrow than we are today.
top of page
Monitoring and Surveillance
How will we know when a pandemic begins? The answer to that key question has driven the search for new and better ways to scan the world for clues that a pandemic influenza virus might be emerging.
In April 2007, the National Institutes of Health (NIH) awarded $161 million to help expand surveillance programs internationally and bolster domestic influenza clinical research, along with research onhow viruses cause disease and how the human immune system responds to influenza viral infections. The CDC has also invested $180 million to help high-risk countries strengthen their capacity to recognize, diagnose, and report influenza outbreaks caused by both avian influenza A (H5N1) and seasonal influenza.
 Diagnostic tests for a range of viruses help doctors and field epidemiologists assess patients for the presence of H5N1, other emerging influenza viruses, and the more common seasonal influenza. In November 2006, the CDC tapped four commercial companies to develop new viral diagnostic tests with quicker and more accurate results. Lab tests for use at the patient’s bedside and at ports of entry and tests designed for large reference and public health laboratories are also being improved.
Diagnostic tests for a range of viruses help doctors and field epidemiologists assess patients for the presence of H5N1, other emerging influenza viruses, and the more common seasonal influenza. In November 2006, the CDC tapped four commercial companies to develop new viral diagnostic tests with quicker and more accurate results. Lab tests for use at the patient’s bedside and at ports of entry and tests designed for large reference and public health laboratories are also being improved.
HHS has also supported State and local health departments as they increase the number of clinicians providing influenza viruses for testing, improve public health laboratory detections, add influenza coordinators in each jurisdiction, and encourage clinicians to test for possible influenza infections.
“The threat to human health will persist as long as the problem persists in animals.”
--Dr. Peter Horby
World Health Organization
February 2005
In January 2007, the CDC in partnership with the Council of State and Territorial Epidemiologists (CSTE) recommended adding “novel influenza viruses”—those with the potential to cause a pandemic—to the list of diseases that States must report to the CDC each week. States and territories have now adopted this recommendation.
Since 2003, HHS has spent $5 million each year to share information with counterpart organizations in Canada and Mexico about urgent infectious disease cases at the three countries’ shared border regions. Recently, border surveillance expanded to include role-playing exercises to test the countries’ responses if airline passengers arrived with possible pandemic influenza.
Genome Sequencing
The HHS Influenza Genome Sequencing Project is an ambitious undertaking to sequence the entire genetic blueprints of 2,250 human and avian influenza viruses from around the world. In February 2007 scientists completed this formidable task and now use this public data to compare different strains of a virus, identify the genetic factors that determine its virulence, and look for new vaccine and diagnostic targets.
International
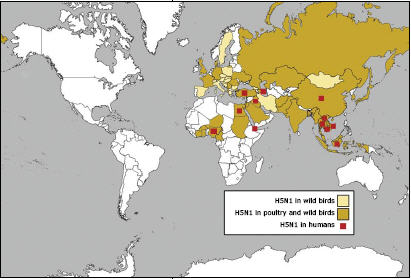 If there is a suspicious die-off of poultry in Egypt or any other country with current cases or significant risk of highly pathogenic avian influenza, it is likely that U.S. public health and animal health specialists monitoring avian influenza outbreaks will quickly learn about it, assess risks and, as needed, jointly pursue appropriate interventions. Considerable effort has gone toward building a strong level of networked international vigilance, which enhances and speeds U.S. awareness of animal disease situations that could lead toward the emergence of a pandemic influenza strain.
If there is a suspicious die-off of poultry in Egypt or any other country with current cases or significant risk of highly pathogenic avian influenza, it is likely that U.S. public health and animal health specialists monitoring avian influenza outbreaks will quickly learn about it, assess risks and, as needed, jointly pursue appropriate interventions. Considerable effort has gone toward building a strong level of networked international vigilance, which enhances and speeds U.S. awareness of animal disease situations that could lead toward the emergence of a pandemic influenza strain.
HHS continues to work with partners throughout the world, including the WHO, to monitor disease spread and prepare for possible pandemics, investing some $125 million in 2006 to fill critical gaps in global disease surveillance and laboratory diagnostic networks. HHS has also established a fund to help local public health authorities quickly move field specimens to reference laboratories for further diagnostic work and confirmation.
HHS continues to call on countries everywhere to support the WHO May 2007 resolution on sharing influenza specimens and viruses within WHO’s Global Influenza Surveillance Network.
top of page
Vaccines and Vaccine Production Capacity
 If an influenza pandemic starts, planners know that six months will elapse before current technology will allow development of a well-matched vaccine. And, after that, rapid large-scale production of this vaccine will not be possible without a significant expansion in domestic manufacturing capacity. Many HHS pandemic preparedness activities work to address that critical six-month period.
If an influenza pandemic starts, planners know that six months will elapse before current technology will allow development of a well-matched vaccine. And, after that, rapid large-scale production of this vaccine will not be possible without a significant expansion in domestic manufacturing capacity. Many HHS pandemic preparedness activities work to address that critical six-month period.
Human H5N1 Vaccine
In April 2007, the Food and Drug Administration (FDA) took an historic step when it approved the first human H5N1 vaccine. If H5N1 or a similar pandemic virus reached the United States, this vaccine could help protect those who would be at the highest risk of exposure to the virus, in the early critical months of a pandemic. Some 12 million doses (enough for 6 million people) have been stockpiled by the U.S. Government. This vaccine is not currently available commercially.
In December 2006, NIH began human trials of another possible vaccine against H5N1 influenza infection. It contains only portions of the influenza virus’s genetic material. Once inside the body, it instructs human cells to make proteins that act as a vaccine. Clinical trials will continue throughout this year.
Cell-Based Vaccine
 The chicken egg has been the critical incubator for many of our influenza-related vaccines for the past half-century. But the high demand for a vaccine during a pandemic calls for a quicker and more flexible technology than the one based on chicken eggs.
The chicken egg has been the critical incubator for many of our influenza-related vaccines for the past half-century. But the high demand for a vaccine during a pandemic calls for a quicker and more flexible technology than the one based on chicken eggs.
Using cells instead of eggs to produce vaccines is already standard practice with many other modern vaccines. This cell-based technology applied to influenza vaccines holds the promise of a reliable and flexible method of producing enough influenza vaccine for every American within six months, following the development of a product that is well-matched to the pandemic viral strain.
HHS will soon ask commercial manufacturers to develop other promising technologies, such as recombinant vaccines, that could further diversify our arsenal. Recombinant vaccines, for example, can be manufactured more quickly than traditional egg-based vaccines.
Vaccine Adjuvant
Adjuvants form another promising technology. Adjuvants are substances added to a vaccine that boost its ability to protect the body from a virus. Added to a vaccine, these adjuvants can help stretch a limited supply by decreasing the amount of vaccine per person while still providing the same level of protection.
 In January 2007, HHS awarded $132.5 million to three vaccine makers to develop adjuvanted vaccines against the H5N1 influenza virus. In addition to allowing immunization with lower doses of vaccine, preliminary results indicate that these adjuvanted vaccines may have the potential to protect against a wider array of influenza viruses. Should further research prove this true, a safe and more effective pre-pandemic vaccine could be developed without having to wait for the emergence of a pandemic virus.
In January 2007, HHS awarded $132.5 million to three vaccine makers to develop adjuvanted vaccines against the H5N1 influenza virus. In addition to allowing immunization with lower doses of vaccine, preliminary results indicate that these adjuvanted vaccines may have the potential to protect against a wider array of influenza viruses. Should further research prove this true, a safe and more effective pre-pandemic vaccine could be developed without having to wait for the emergence of a pandemic virus.
Expanding Capacity
Over the next five years, vaccine manufacturers, spurred by a $1 billion HHS investment, will develop the capacity to produce and deliver enough pandemic vaccine for every man, woman, and child in the United States within six months of the appearance of a pandemic virus. More funding will follow in the near future to help build U.S. factories that will produce cell-based influenza vaccine.
International
In many countries, national vaccine production capacity cannot meet the needs of its citizens. To help extend protections to our global neighbors, HHS provided $10 million to the WHO, which then awarded that sum to six key developing countries in April 2007 to expand their local infrastructure and produce safe, effective seasonal influenza vaccines. This expanded capacity would also help fill their own (and possibly regional) needs in the event of a pandemic.
top of page
Antiviral Drugs
 In January 2007, scientists around the world held their collective breath as early news from Egypt indicated that the avian influenza virus that had killed two human victims there seemed to have developed resistance to the antiviral drug oseltamivir, one of only two antiviral drugs that combat most current influenza viruses. Further testing showed that the resistance was similar to what had already been noted two years earlier in Thailand and that resistant viruses were not spreading. But the experience underscored the critical role played by antiviral medications in helping reduce pandemic influenza symptoms and shorten the length of illness.
In January 2007, scientists around the world held their collective breath as early news from Egypt indicated that the avian influenza virus that had killed two human victims there seemed to have developed resistance to the antiviral drug oseltamivir, one of only two antiviral drugs that combat most current influenza viruses. Further testing showed that the resistance was similar to what had already been noted two years earlier in Thailand and that resistant viruses were not spreading. But the experience underscored the critical role played by antiviral medications in helping reduce pandemic influenza symptoms and shorten the length of illness.
HHS antiviral drug strategy includes supporting the development of new drugs that can broaden our ability to treat and even prevent influenza as well as stockpiling existing antiviral drugs.
New Antiviral Drugs
In January 2007, HHS awarded some $103 million to develop a new influenza antiviral drug, peramivir, which has proven effective in laboratory tests against a number of influenza strains. Further studies will test whether this drug can treat seasonal and other life-threatening influenza viruses such as H5N1.
Stockpiling Antiviral Drugs
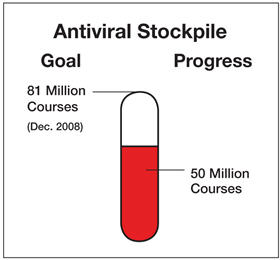
 HHS, working closely with State governments, is striving toward the goal of having 81 million antiviral treatment courses on hand: 50 million by HHS for distribution to States through the Strategic National Stockpile when an influenza pandemic is judged imminent and 31 million by the States for their respective stockpiles. A treatment course is enough medication to treat one person. This antiviral drug supply will allow public health officials to help contain or suppress a pandemic outbreak. The 81 million antiviral treatment courses include six million treatment courses set aside for the early stages of an emerging pandemic.
HHS, working closely with State governments, is striving toward the goal of having 81 million antiviral treatment courses on hand: 50 million by HHS for distribution to States through the Strategic National Stockpile when an influenza pandemic is judged imminent and 31 million by the States for their respective stockpiles. A treatment course is enough medication to treat one person. This antiviral drug supply will allow public health officials to help contain or suppress a pandemic outbreak. The 81 million antiviral treatment courses include six million treatment courses set aside for the early stages of an emerging pandemic.
HHS has purchased or has at hand approximately 36 million antiviral treatment courses. Almost all States have taken advantage of a Federal discount plan and, to date, have purchased more than 12 million treatment courses.
Stockpiling Other Supplies
 Along with the antiviral drugs, other types of equipment and material help reinforce the HHS and State stockpiles. These will help prevent people’s direct contact with the pandemic virus, when it emerges, and reduce the spread of the disease. HHS has purchased over 155 million surgical masks and respirators; this total includes 104 million N95 respirators and 52 million surgical masks. HHS has also committed $100 million to buy ventilators, syringes, and intravenous antibiotics and to store and secure these supplies for pandemic-affected areas.
Along with the antiviral drugs, other types of equipment and material help reinforce the HHS and State stockpiles. These will help prevent people’s direct contact with the pandemic virus, when it emerges, and reduce the spread of the disease. HHS has purchased over 155 million surgical masks and respirators; this total includes 104 million N95 respirators and 52 million surgical masks. HHS has also committed $100 million to buy ventilators, syringes, and intravenous antibiotics and to store and secure these supplies for pandemic-affected areas.
International
As a supplement to the national stockpile, HHS has prepositioned overseas stockpiles of personal protective equipment, decontamination kits, and antiviral medications to complement global efforts to help contain pandemic influenza overseas.
top of page
State and Local Preparedness
Five thousand communities across the nation have actively joined forces with State and Federal government officials to practice and improve ways they can work together to manage a pandemic influenza.
“The more informed we are, the more empowered we are.”
--Participant at Pandemic Preparedness Forum
June 13, 2007
Planning
 By December 2006, with $325 million in funding from HHS, States, territories, and tribes had held 59 pandemic influenza planning summits, where Federal, State, and local leaders; health professionals; schools; and the private sector could begin to work together toward resolving State and community preparedness issues.
By December 2006, with $325 million in funding from HHS, States, territories, and tribes had held 59 pandemic influenza planning summits, where Federal, State, and local leaders; health professionals; schools; and the private sector could begin to work together toward resolving State and community preparedness issues.
HHS provided another $250 million to intensify ongoing pandemic preparedness exercises. These include incorporating community mitigation measures, containment plans, the medical surge capacity to treat influenza victims, and seasonal influenza vaccinations to test mass inoculation capabilities.
As a follow-up, HHS asked the National Governors Association (NGA) to hold 10 regional pandemic influenza workshops this year to test intergovernmental and interstate coordination.
Internationally, HHS has provided onsite technical assistance to help more than 20 countries develop their own national pandemic preparedness plans. These reflect the best science on preparing for and responding to a potential pandemic.
Medical Surge
If a pandemic strikes a community, its hospitals, clinics, and other health care delivery sites could quickly reach their capacity to provide critical care to influenza victims. Having a plan to draw on other local resources is one way to help gain control of that situation. HHS has given both funding and guidance to community health officials throughout the country so they can strengthen their local medical surge capabilities.
Local groups have worked through such critical issues as the purchase and storage of pharmaceutical caches (including antiviral drugs for health care personnel and their families), isolation capacity, plans to provide care at alternate sites, stockpiling personal protective equipment (PPE) and ventilators, developing bed-tracking systems, buying and testing interoperable communications, and conducting pandemic influenza exercises and training.
Facemasks and Respirators
 One important question was whether to use or not use a facemask or respirator. An answer came in May 2007 when the CDC presented preliminary advice to the public about using facemasks and respirators in certain public (nonwork) settings during an influenza pandemic.
One important question was whether to use or not use a facemask or respirator. An answer came in May 2007 when the CDC presented preliminary advice to the public about using facemasks and respirators in certain public (nonwork) settings during an influenza pandemic.
This interim guidance stresses that in a pandemic situation surgical masks and respirators may help prevent or slow the spread of the pandemic when they are used along with hand washing, avoiding people who have influenza, treating those infected with antiviral medications, having people who care for ill family members voluntarily stay home, and encouraging people to avoid crowded places and large gatherings.
Very little research now exists about the usefulness of surgical masks or respirators, so studies are under way to learn more about whether surgical masks and/or respirators can provide protection from influenza and how people should consider using them.
FDA has approved two N95 respirators that people can use in a public health emergency to help reduce their exposure to airborne germs.
“To be truly prepared, all leaders and individuals across communities must be involoved.”
--Assistant Secretary John Agwunobi, HHS
June 13, 2007
Community Mitigation
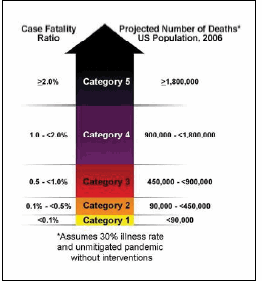 In February 2007, the CDC released a community mitigation interim guidance to help local communities make appropriate decisions about what actions to take to help delay or mitigate the spread of a pandemic, and when to take those actions. These community mitigation actions would be especially critical in the first six months of a pandemic. The interim guidance introduced a Pandemic Severity Index (PSI), akin to the National Weather Service’s hurricane intensity scale. Both scales move up from 1 to 5 as the severity of the situation increases.
In February 2007, the CDC released a community mitigation interim guidance to help local communities make appropriate decisions about what actions to take to help delay or mitigate the spread of a pandemic, and when to take those actions. These community mitigation actions would be especially critical in the first six months of a pandemic. The interim guidance introduced a Pandemic Severity Index (PSI), akin to the National Weather Service’s hurricane intensity scale. Both scales move up from 1 to 5 as the severity of the situation increases.
The Pandemic Severity Index links information about the severity of disease spread (number of fatalities) to specific measures that could be implemented. These measures range from encouraging individuals to stay home voluntarily when they become ill, to more stringent “social distancing” measures such as closing schools and canceling public gatherings. By quickly adding these multiple actions, communities could help balance the need to protect the public’s health and the need to minimize a pandemic’s social and economic disruptions.
Many Federal, State, and local governments are incorporating this community mitigation guidance into their pandemic planning processes, and they are distributing the guidance throughout their systems. The Community Strategy for Pandemic Influenza Mitigation is available at http://www.pandemicflu.gov/plan/community/commitigation.html.
Vaccine Allocation
 In a pandemic, until there is enough vaccine for everyone, determining which individuals and groups should have early access to available vaccines is one of the thorniest problems that Federal, State, local, tribal, and territorial pandemic planners face.
In a pandemic, until there is enough vaccine for everyone, determining which individuals and groups should have early access to available vaccines is one of the thorniest problems that Federal, State, local, tribal, and territorial pandemic planners face.
The Federal government is soliciting advice from influenza experts, State and local public health officials, homeland security experts, ethicists, private-sector stakeholders, and the public. They identified the most important vaccination goals as protecting those who will first respond to the pandemic and care for the sick, those who keep vital community services working, those who have increased exposure due to their jobs, and children.
top of page
Communications
Reporters, government officials, and local airport quarantine officers gather around a table to role-play a scenario that imagines the arrival of a passenger in the early stages of pandemic influenza. What does the quarantine officer say to the passenger? What if the passenger refuses to cooperate? How does the officer explain the situation to local reporters? What does the reporter report? What do the government officials say to national reporters?
These and other challenging questions arose during six recent tabletop exercises that brought together both key media leaders and senior government officials in New York; Chicago; Los Angeles; Miami; Washington, D.C.; and Atlanta. In their dialogues, the two groups considered the challenges they would face and what could be done to address them in a public health crisis.
“An influenza pandemic knows no political or geographic boundaries.”
--Assistant Secretary Craig Vanderwagen, HHS
November 14, 2006
HHS pandemic communications planning is built on the principle that every person in the country needs—and should get—immediate and accurate information about the rapidly changing pandemic situation. And HHS is building networks of communicators, within and outside the Federal government and within and outside our country, to help make sure that needed information is available to all.
 In February 2007, HHS launched a series of television and radio public service announcements (PSAs) in English and Spanish to raise awareness of pandemic influenza and to educate the public about the steps people can take now to prepare. The PSAs, released under the title Know What to Do about Pandemic Flu, were distributed to 300 television and 1,000 radio stations across the country.
In February 2007, HHS launched a series of television and radio public service announcements (PSAs) in English and Spanish to raise awareness of pandemic influenza and to educate the public about the steps people can take now to prepare. The PSAs, released under the title Know What to Do about Pandemic Flu, were distributed to 300 television and 1,000 radio stations across the country.
In June 2007, HHS convened an interactive Leadership Forum for influential leaders from the business, faith, civic, and health care sectors to participate in dynamic discussions aimed at helping Americans become more prepared for a possible pandemic. In conjunction with the Forum, HHS launched a five-week-long “blog summit” —an online event—that allowed leaders and members of the public to join an open conversation and begin to shape thinking about how to communicate the critical need for preparedness at home and within workplaces and communities.
HHS has worked closely with Federal, State, tribal, and territorial communications specialists to develop plans that will help guide the public, the news media, health care providers, and other groups to respond appropriately in outbreak situations and to encourage people to follow the necessary public health measures. A government-wide public health emergency communications plan is now ready to activate in the event of a pandemic. The website www.pandemicflu.gov, managed by HHS on behalf of the entire Federal government, continues to provide one-stop access to all Federal avian and pandemic influenza information. The entire site has been translated into Spanish, and key documents are also available in Chinese and Vietnamese.
 To help know what planning questions to ask, HHS has developed and distributed 19 checklists and guidances. State and local governments, businesses, school systems, colleges and universities, health care organizations, and individuals and families can draw on these checklists and guidances as they lay their own plans. Many of the actions are specific to pandemic influenza, but some are useful for any public health emergency. All are available at www.pandemicflu.gov.
To help know what planning questions to ask, HHS has developed and distributed 19 checklists and guidances. State and local governments, businesses, school systems, colleges and universities, health care organizations, and individuals and families can draw on these checklists and guidances as they lay their own plans. Many of the actions are specific to pandemic influenza, but some are useful for any public health emergency. All are available at www.pandemicflu.gov.
Internationally, HHS has provided expertise and funding to the WHO for training and exercises for local communications officials and journalists.
top of page
Timeline—Pandemic Planning Update IV
April - November 2005
April 2005 – RFP for cell-based vaccine contract issued
November 2005 – President Bush announces the National Strategy for Pandemic Influenza
Summer 2005 – Preliminary results from H5N1 vaccine clinical trials indicate immune response predictive of protection against H5N1
September 2005 – International Partnership on Avian and Pandemic Influenza launched
November 2005 – HHS releases Pandemic Influenza Plan
October 2005 – Southeast Asia fact-finding mission led by HHS Secretary and State Department
December 2005
December 2005 – Passage of the Public Readiness and Emergency Preparedness Act (PREP Act)
December 2005 – Enrollment completed for clinical trials with H5N1 vaccine in older healthy adults
December 2005 – Convening of States Summit in Washington, DC
December 2005 – Release of State and Local and Business Checklists
December 2005 – First State Summit in Minnesota
December 2005 –Tabletop communications exercises with US Departments and representatives from Canada and the UK
January 2006
January 2006 – RFI issued on increasing egg-based vaccine capacity
January 2006 – Bilateral teams sent to Turkey, Armenia, Azerbaijan, Georgia, Romania, Ukraine and Nigeria to assess avian flu outbreaks
January 2006 – International Pledging Conference in Beijing; $334M in US grants and technical assistance to aid global effort
January 2006 – 6 State summits in AZ, VT, WV, RI, GA, KY
January 2006 – RFI issued on advanced development of promising antivirals
January 2006 – Release of Individuals and Families, Faith-based and Community Organizations, and School Districts (K-12) Checklists
February 2006
February 2006 – Enrollment completed for pediatric H5N1 vaccine clinical trial
February 2006 – Risk Communications Preparedness Workshop hosted by CDC in Thailand
February 2006 – 11 State Summits in CT, IA, MA, FL, OH, NV, DE, AL, MO, NE, MD
February 2006 – Laboratory assay for diagnostic testing of avian influenza A/H5 approved
March 2006
March 2006 – Release of Medical Offices and Clinics, Home Health Care, Child Care and Preschool, and Colleges and Universities Checklists
March 2006 – Pandemic flu supplemental guidance sent to states
March 2006 – 19 State Summits in SC, SD, ND, WY, WI, PA, IL, NC, VA, IN, PR, CO, UT, ID, TX, NM, OR, CA and the Virgin Islands
March 2006 – Published results demonstrate that inactivated H5N1 vaccine induces immune responses in healthy adults
March 2006 – RFP issued for advanced development of antigen sparing technologies
March 2006 – FDA approves Relenza for the prevention of Influenza A and B in adults and children
March 2006 – States receive initial grant funding for pandemic preparedness
March 2006 – Order of 16.2 million courses of Tamiflu and 3.9 million courses of Relenza
April 2006
April 2006 – 7 State Summits in TN, AK, WA, HI, LA, MI, and the District of Columbia
April 2006 – Government leaders from the Freely Associated States and Pacific Territories participate in the Hawaii and Guam summits
April 2006 – FDA issues guidance for industry on diagnostic devices to detect influenza A viruses
April 2006 – WHO issues guidelines for humanitarian agencies
April 2006 – NIH researchers model spread of influenza through United States by virus type, size of population and commuting rates and distance
April 2006 – HHS provides assistance to Bangladesh and India to enhance planning for a potential influenza pandemic
May 2006
May 2006 –Release of Homeland Security Council National Strategy for Pandemic Influenza: Implementation Plan
May 2006 – HHS awards contracts totaling more than $1 billion to develop cell-based influenza vaccine
May 2006 – Department of State establishes Avian Influenza Action Group to coordinate US international effort
May 2006 – Release of Long-Term Care and Other Residential Facilities Checklist
May 2006 – 7 State Summits in MS, OK, NJ, KS, NH, MT, and Tribal Leaders
May 2006 – HHS Secretary addresses the World Health Assembly on pandemic flu preparedness
June 2006
June 2006 – Secretaries Leavitt of HHS, Norton of DOI, and Johanns of USDA hold national briefing on US preparations for avian influenza
June 2006 – HHS Secretary addresses health ministers of Central America on pandemic preparedness
June 2006 – CDC issues updated Interim Guidance for Laboratory Testing of Persons with Suspected Infection with Avian Influenza A (H5N1) virus in the US
June 2006 – Indonesia holds H5N1 influenza expert consultation
June 2006 – Congress passes $2.3 billion funding for pandemic preparedness
July 2006
July 2006 – 2 Summits in Arkansas and New York City
July 2006 – HHS allocates $170 million to subsidize state purchases of antiviral drugs
August 2006
Aug 2006- Quick diagnosis of flu strains possible with new microchip test
August 2006 – State Summit in New York
September 2006
Sep 2006- FDA issues guidance to industry for development of cell-based viral vaccines
October 2006
October 2006 – Guidance on use of surgical masks and respirators in health care settings released
October 2006 – WHO issues global influenza action plan to increase vaccine supply
November 2006
November 2006 – United States and Mexico pledge increased cooperation in pandemic influenza preparedness along border
November 2006 – HHS awards $199 million to manufacturers for 5.3 million doses of H5N1 vaccine
November 2006–WHO issues guidelines for investigating human cases of avian flu
November 2006—HHS taps four commercial companies to develop new H5N1 diagnostic tests
December 2006
December 2006–CDC awards $11.4 million for rapid bird flu diagnostic tests
December 2006—NIH begins human clinical trials of DNA-based H5N1 vaccine
December 2006–Template for tabletop planning exercises released
January 2007
January 2007–CDC adds “pandemic flu-like viruses” to list of reportable diseases
January 2007—HHS awards $132.5 million to develop H5N1 adjuvanted influenza vaccines
January 2007–HHS awards $102.6 million for advanced development of new influenza antiviral drug, peramivir
January 2007–U.S. Businesses with Overseas Operations checklist released
January 2007–Vaccine for H5N1 influenza enters human clinical trials
February 2007
February 2007 – WHO and OIE Revise Influenza Vaccine Development Guidance
February 2007–HHS launches pandemic flu public service announcements
February 2007–CDC releases community mitigation guidance
February 2007–2,250 human and avian influenza virus genomes completed and publicly accessible
March 2007
March 2007–Health Insurers Planning Checklist released
April 2007
April 2007–FDA approves first US H5N1 human influenza vaccine
April 2007–12 million doses of human H5N1 vaccine added to Strategic National Stockpile
April 2007–HHS provides $10 million to WHO to expand vaccine infrastructure in six developing countries
April 2007–Travel Industry Planning Checklist released
April 2007–NIH awards $161 million to expand surveillance and bolster research
May 2007
May 2007—HHS issues interim guidance on community (non-work) use of facemasks
May 2007 – FDA clears first respirators for public health emergencies
May 2007 – New online training course released for state and local public health responders
May 2007 – Pandemic Flu Leadership Blog begins
May 2007 – World Health Assembly agreement reached on influenza virus sharing
May 2007 – FDA finalizes guidances for manufacturers of pandemic and seasonal flu vaccines
June 2007
June 2007 – WHO moves ahead with plans for global stockpile of H5N1 vaccine
June 2007 – HHS convenes Pandemic influenza Leadership Forum for faith-based, civic and health care leaders to encourage preparedness
top of page