


FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
![]()
   |
| FDA
Home Page | CDRH Home Page | Search
| CDRH
A-Z Index | Contact CDRH
|
  |
| Information
for mammography facility personnel, inspectors, and consumers about the implementation of the Mammography Quality Standards Act of 1992 (MQSA) |
The Division of Mammography Quality and Radiation Programs (DMQRP) within the Food and Drug Administration (FDA) celebrated an important anniversary earlier this year: the 10 th anniversary of the first mammography facility inspection under the Mammography Quality Standards Act (MQSA). The first inspection was preformed on January 16, 1995 . During the height of winter weather in the northern plains, Warren Freier, a newly trained and FDA-certified MQSA inspector with the North Dakota Radiation Control Program, drove through a raging snow storm in blizzard conditions to reach his appointment with Meritcare Medical Group in Fargo to perform this first inspection. Upon arrival, Warren reported, he received a much needed warm reception!
And how did the inspection turn out? Warren said it went well. Virginia Moon, Director of Meritcare's Mammography Services said, "We were a little apprehensive about the inspection, but we weren't terribly concerned because we've always tried to do high quality mammography. Our previous experience with the American College of Radiology accreditation gave us a good idea of what to expect."

Warren and facility staff during the first inspection: Left to right: S. Brent Colby, M.S., physicist,
Maria Chamberlain, R.T. (M), staff technologist, and Warren Freier, inspector.
More MQSA inspections quickly followed and by late March 1995, FDA had uploaded data from more than 300 inspections into its computerized database from across the country. To date, MQSA inspectors have completed a total of nearly 93,000 facility inspections.
MQSA Inspectors
Mammography facility inspections performed by FDA-qualified personnel are an integral component of the MQSA program. Today, more than 10 years since the inception of FDA's MQSA inspector training, DMQRP has trained a total of 436 inspectors. Currently there are 237 active FDA-certified MQSA inspectors; 203 are State inspectors and the remaining 34 are FDA inspectors.
Prior to beginning MQSA training, FDA requires inspectors to meet specific qualifications. Mammography inspector training consists of the successful completion of Courses 1, 2, and 3. Each course is two weeks in length, although the first course is now offered online. The Course's contents include:
MQSA inspectors have proven to be dedicated professionals serious about ensuring that quality standards are met. Our inspectors' hard work and commitment to the MQSA goal of quality mammography nationwide has been remarkable. Starting with each inspector's successful completion of the rigorous training and continuing through their performance of annual inspections for each mammography facility within their charge, MQSA inspectors and the entire inspection program have indeed proven to be a vital part of the MQSA story.
Facility Inspections
By reviewing the inspection data within MPRIS, FDA has identified and evaluated trends in inspection findings over the past 10 years. The following chart shows the increase in the number of facilities without any adverse inspection observations over the course of the program.
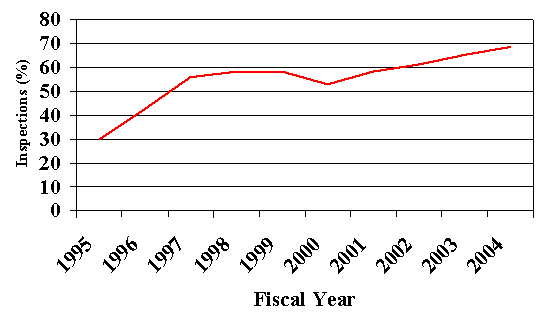
| 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 |
| 30 | 42.7 | 55.9 | 58.2 | 58.4 | 53 | 58.3 | 61.4 | 65.6 | 68.8 |
Note: In 1999, FDA started inspecting facilities under the final regulations. This resulted in a decline in the number of observation-free inspections for the remainder of 1999 and during 2000 while facilities adjusted to new requirements. Once this period passed, the overall trend toward facility compliance continued.
On the Horizon
Although the program currently has a full complement of inspectors, the MQSA inspector training program is still active, offering Courses 1, 2 and 3 on a yearly basis. As with all jobs, our program has experienced some inspectors moving on to other career opportunities, some have retired, and sadly, a few inspectors have passed away. For DMQRP, it is an ongoing job to keep our nationwide cadre of inspectors complete. Just like it was at the time of the first inspections 10 years ago, DMQRP values the efforts and hard work of our mammography inspectors. With pride we thank them all for their dedication to the program -- both those currently active and those now retired -- those with many years of experience and those newly trained and recently qualified.
DMQRP is continually reviewing the many aspects involved in administering this nationwide program. With the reassessment processes, the division has an eye to the future.We evaluate and consider whatrefinements or improvements are needed and in which areas: compliance activities; our partnership with the accreditation bodies and the States-As-Certifiers program; new modalities in mammography such as full field digital; and our inspection electronic data systems, just to name a few.
In 2005, ten years after the first mammography facility inspection was performed, FDA's goal for the MQSA program remains, "To improve the quality of the nation's mammography services." Toward that principle, DMQRP continues to work, keeping in mind that we hope to achieve the goal of affording quality mammography services for all of our nation's women via an ever efficient and least burdensome approach to facilities.
Updated May 24, 2005
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH