Back to: About NCCAM : Office of the Director
Director's Page
Josephine P. Briggs, M.D.
Message from the Director
CAM Research
June 3, 2008
I am now 4 months into this exciting and challenging new job as Director of NCCAM. In this short period, I have been given an excellent orientation to all areas of responsibility of the Center, guided by our terrific staff. I have also had the opportunity to speak with many CAM investigators, practitioners, and thought leaders, and some of our critics as well. I am learning a great deal from these various communities and perspectives. Clinicians have given me a sense of the promise that they see in the CAM approaches to health and wellness. Scientists have discussed the many challenges and knowledge gaps in conducting CAM research as well as the opportunities that are emerging from new research methods. NCCAM staff members have shared with me their expertise and experience in the complexities of oversight of our highly varied research portfolio. And CAM advocates who closely follow our progress have shared their perspective and hopes for NCCAM as well.
Like much of modern science, CAM research requires good team work and many kinds of expertise. I am confident that the questions are important ones— scientifically interesting and of substantial potential public health impact. I am also convinced that new methods for basic and clinical research are creating new opportunities of particular value for studies about CAM. We can make major strides to strengthen the knowledge base about safety and efficacy of CAM and identify ways that CAM can make a valuable and valued contribution to the health and well being of the public.
To organize and structure the types of research that NCCAM supports, I am finding it helpful to utilize the framework shown schematically below to describe our research enterprise. Each of these components is critical to understanding CAM; by and large, for each promising CAM modality we need to support research in each of these categories to build a strong evidence base. Only when that evidence base is constructed will we have the information that the public and practitioners need.
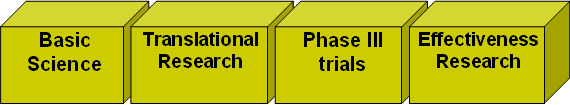
For each type of CAM therapy, we can ask:
BOX 1: Basic Research. What are the possible underlying biological mechanisms of this therapy?
- Basic studies that investigate the physiological changes that result from a CAM therapy and all potential biological effects that might have an impact on health, including placebo effects.
- Chemistry, molecular biology, cellular, whole animal, and human subjects studies that build the evidence for biological plausibility.
BOX 2: Translation. Do we have the understanding and needed methodological tools to perform definitive human subject studies on this therapy? Do we have adequate 'proof of concept' data to justify the investment?
- Understanding dosage and bioavailability
- Development of surrogate markers that establish that the intervention has an effect
- Early phase human studies that provide a 'proof of concept' of benefit.
BOX 3: Randomized Clinical Trials. Do we have definitive studies that test the efficacy of this therapy for specific indications on well-defined populations?
- Randomized controlled trials, usually double-blinded, to study the efficacy of a CAM therapy in a particular disease application on a well-defined population.
BOX 4: Effectiveness Studies: What are the patterns of use and observed effects of this CAM therapy in the real world? How effective is CAM in promoting patient well being in real-world applications?
- Epidemiology and natural history cohort studies.
- Randomized trials that assess real-world effectiveness; for example, a study might compare patient outcomes with standard medical care and with the addition of access to a CAM modality.
- Studies of the context of care, including determining the effect of provider-patient interactions.
Clearly, this is a big undertaking, and we face challenging fiscal realities. Our priority setting needs to take into account both scientific promise and the public interest in CAM interventions. We plan to turn to our Advisory Council for help in defining priorities and to continue to seek venues to get input from all our stakeholders. We anticipate much further discussion. As I continue the dialogue with all our communities, I welcome your thoughts.
Josephine P. Briggs, M.D.
Director
National Center for Complementary and Alternative Medicine
National Institutes of Health
To contact Dr. Briggs, email nccamdirector-r@mail.nih.gov
Back to the Current Message From The Director
Note: The PDF file requires a viewer such as Adobe Reader, which you can download free of charge from the Adobe Web site.
