Page Options
Media:
Contact Us
- 800–CDC–INFO
- (800-232-4636)
- 888–232–6348 (TTY)
- cdcinfo@cdc.gov
Weekly Report: Influenza Summary Update
2008-2009 Influenza Season Week 52 ending December 27, 2008
(All data are preliminary and may change as more reports are received.)Synopsis:
During week 52 (December 21-27, 2008), increased influenza activity was reported in the United States.
- One hundred thirty (5.0%) specimens tested by U.S. World Health Organization (WHO) and National Respiratory and Enteric Virus Surveillance System (NREVSS) collaborating laboratories and reported to CDC/Influenza Division were positive for influenza.
- The proportion of deaths attributed to pneumonia and influenza (P&I) was below the epidemic threshold.
- One pediatric influenza-associated death was reported.
- The proportion of outpatient visits for influenza-like illness (ILI) was below the national baseline. The South Atlantic region reported ILI at their region-specific baseline.
- Three states reported regional activity; 10 states reported local influenza activity; the District of Columbia and 30 states reported sporadic influenza activity; six states reported no influenza activity; and one state did not report.
Region |
Data for current week | Data cumulative for the season | ||||||
|---|---|---|---|---|---|---|---|---|
| Out-patient ILI* | % positive for flu† | Number of jurisdictions reporting regional or widespread activity‡ | A (H1) | A (H3) | A Unsub-typed | B | Pediatric Deaths | |
| Nation | Normal | 5.0 % | 3 of 51 | 301 | 33 | 470 | 193 | 1 |
| New England | Normal | 1.3 % | 1 of 6 | 3 | 1 | 14 | 3 | 0 |
| Mid-Atlantic | Normal | 2.4 % | 1 of 3 | 20 | 3 | 14 | 6 | 0 |
| East North Central | Normal | 6.0 % | 0 of 5 | 13 | 2 | 12 | 6 | 0 |
| West North Central | Normal | 0.8 % | 0 of 7 | 6 | 0 | 18 | 7 | 1 |
| South Atlantic | Normal | 3.6 % | 1 of 9 | 18 | 0 | 107 | 79 | 0 |
| East South Central | Normal | 0.8 % | 0 of 4 | 1 | 0 | 0 | 2 | 0 |
| West South Central | Normal | 8.4 % | 0 of 4 | 55 | 0 | 193 | 68 | 0 |
| Mountain | Normal | 4.4 % | 0 of 8 | 14 | 21 | 36 | 6 | 0 |
| Pacific | Normal | 3.3 % | 0 of 5 | 171 | 6 | 76 | 16 | 0 |
* Elevated means the % of visits for ILI is at or above the national or
region-specific baseline
† National data is for current week; regional data is for the most recent three weeks.
‡ Includes all 50 states and the District of Columbia
U.S. Virologic Surveillance:
WHO and NREVSS collaborating laboratories located in all 50 states and Washington D.C. report to CDC the number of respiratory specimens tested for influenza each week. Results of these tests performed during the current week and cumulative totals for the season are summarized in the table below.
| Week 51 | Cumulative for the Season | |
|---|---|---|
| No. of specimens tested | 2,610 | 45,389 |
| No. of positive specimens (%) | 130 (5.0%) | 997 (2.2%) |
| Positive specimens by type/subtype | ||
| Influenza A | 105 (80.8%) | 804 (80.6%) |
| A (H1) | 18 (17.1%) | 301 (37.4%) |
| A (H3) | 4 (3.8%) | 33 (4.1%) |
| A (unsubtyped) | 83 (79.1%) | 470 (58.5%) |
| Influenza B | 25 (19.2%) | 193 (19.4%) |
The District of Columbia and forty-one states from all nine surveillance regions have reported laboratory-confirmed influenza this season with three states accounting for 722 (72.4%) of the 997 reported influenza viruses.
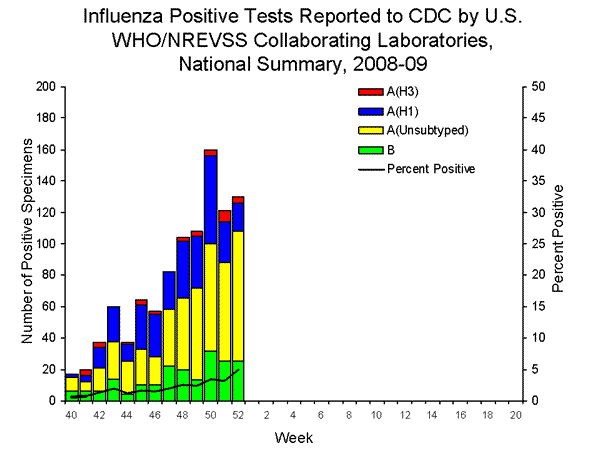
View WHO-NREVSS Regional Bar Charts| View Chart Data | View Full Screen
Antigenic Characterization:
CDC has antigenically characterized 104 influenza viruses [68 influenza A (H1N1), seven influenza A (H3N2) and 29 influenza B viruses] collected by U.S. laboratories since October 1, 2008.
All 68 influenza A (H1N1) viruses are related to the influenza A (H1N1) component of the 2008-09 influenza vaccine (A/Brisbane/59/2007). All seven influenza A (H3N2) viruses are related to the influenza A (H3N2) vaccine component (A/Brisbane/10/2007).
Influenza B viruses currently circulating can be divided into two distinct lineages represented by the B/Yamagata/16/88 and B/Victoria/02/87 viruses. Nine influenza B viruses tested belong to the B/Yamagata lineage and are related to the influenza B vaccine component (B/Florida/04/2006). The remaining 20 viruses belong to the B/Victoria lineage and are not related to the influenza B vaccine strain. Seventeen of the 20 viruses belonging to the B/Victoria lineage were submitted from two states.
Data on antigenic characterization should be interpreted with caution given that:
- Few U.S. isolates are available for testing because of limited influenza activity thus far.
- The majority of viruses antigenically characterized to date come from only three states and may not be nationally representative.
- Antigenic characterization data is based on hemagglutination inhibition (HI) testing using a panel of reference ferret antisera and results may not correlate with clinical protection against circulating viruses provided by influenza vaccination
Annual influenza vaccination is expected to provide the best protection against those virus strains that are related to the influenza vaccine strains, but limited to no protection may be expected when the vaccine and circulating virus strains are so different as to be from different lineages, as is seen with the two lineages of influenza B viruses.
Antiviral Resistance:
Since October 1, 2008, 73 influenza A (H1N1), 11 influenza A (H3N2), and 33 influenza B viruses from 21 states have been tested for resistance to the neuraminidase inhibitors (oseltamivir and zanamivir). More than half (58%) of these tested viruses tested were from only three states. Seventy-three influenza A (H1N1) and 11 influenza A (H3N2) viruses from 18 states have been tested for resistance to the adamantanes (amantadine and rimantadine). More than half (56%) of these tested viruses tested were from only three states as well. The results of antiviral resistance testing performed on these viruses are summarized in the table below.
| Isolates tested (n) | Resistant Viruses, Number (%) |
Isolates tested (n) | Resistant Viruses, Number (%) | ||
|---|---|---|---|---|---|
| Oseltamivir | Zanamivir | Adamantanes | |||
| Influenza A (H1N1) | 73 | 72 (99%) | 0 (0) | 73 | 0 (0%) |
| Influenza A (H3N2) | 11 | 0 (0) | 0 (0) | 11 | 11 (100%) |
| Influenza B | 33 | 0 (0) | 0 (0) | N/A* | N/A* |
With low levels of influenza activity thus far in the 2008-09 season in the United States, overall numbers of virus specimens and the number of states that have submitted specimens for testing is limited. The limited number and geographic diversity of specimens tested for antiviral resistance, as well as the uncertainty regarding which influenza virus types or subtypes will predominate during the season, make it too early to make an accurate determination of the prevalence of influenza viruses resistant to oseltamivir nationally or regionally at this time. CDC has solicited a representative sample of viruses from WHO collaborating laboratories in the United States, and more specimens are expected as influenza activity increases.
Pneumonia and Influenza (P&I) Mortality Surveillance
During week 52, 6.8% of all deaths reported through the 122-Cities Mortality Reporting System were due to P&I. This percentage is below the epidemic threshold of 7.5% for week 52.
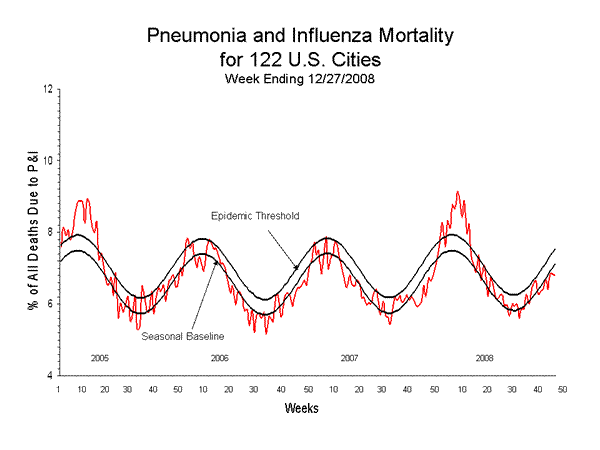
View Full Screen
Influenza-Associated Pediatric Mortality
One influenza-associated pediatric death was reported to CDC from Minnesota during week 52. This death occurred during week 51 (the week ending December 20, 2008). This was the first reported influenza-associated pediatric death occurring during the 2008-09.
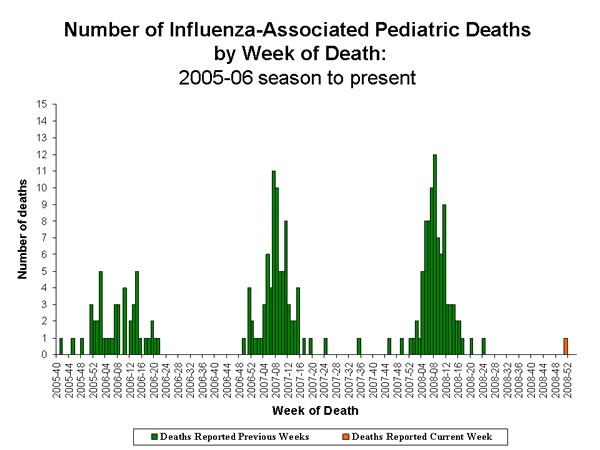
View Full Screen
Influenza-Associated Hospitalizations
Laboratory-confirmed influenza-associated hospitalizations are monitored in two population-based surveillance networks: the Emerging Infections Program (EIP) and the New Vaccine Surveillance Network (NVSN).
No influenza-associated hospitalizations have been reported from the New Vaccine Surveillance Network this season.
During October 1 – December 20, 2008, preliminary laboratory-confirmed influenza-associated hospitalization rates reported by the EIP for children aged 0-4 years and 5-17 years were 0.3 per 10,000 and 0.01 per 10,000, respectively. For adults aged 18-49 years, 50-64 years, and = 65 years, the rates were 0.03 per 10,000, 0.04 per 10,000, and 0.1 per 10,000, respectively.
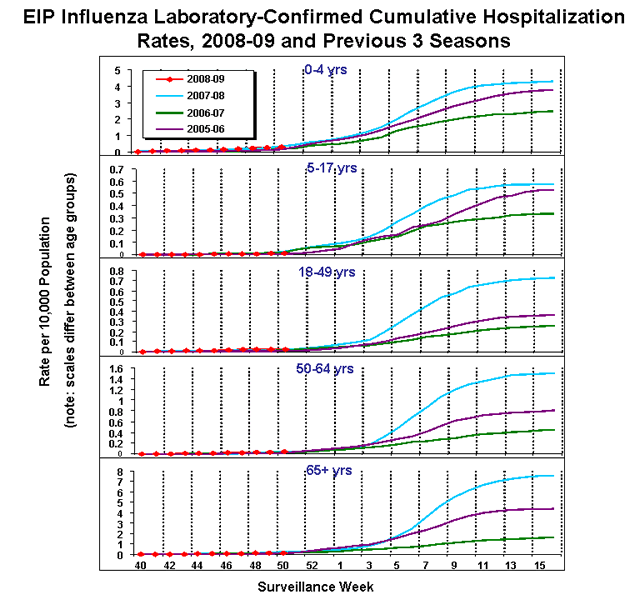
View Full Screen
Outpatient Illness Surveillance:
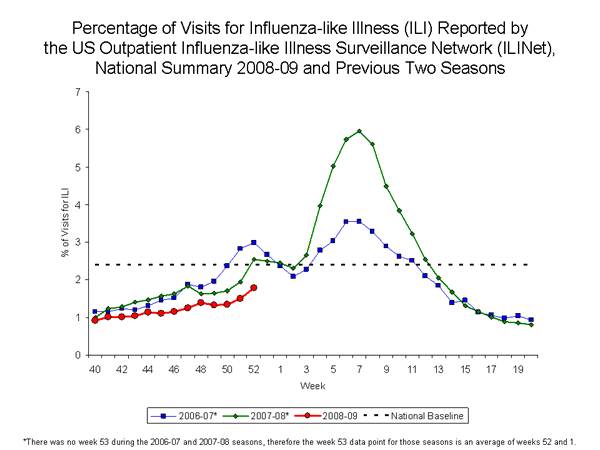
During week 52, 1.8% of patient visits reported through the U.S. Outpatient Influenza-like Illness Surveillance Network (ILINet) were due to influenza-like illness (ILI). This percentage is less than the national baseline of 2.4%. On a regional level, the percentage of visits for ILI ranged from 0.4% to 2.4%. One region (South Atlantic) reported 2.2% of outpatient visits for ILI, which is equal to their region-specific baseline of 2.2%, while the remaining eight regions reported percentages of visits for ILI below region-specific baseline levels.
View Sentinel Providers Regional Charts | View Chart Data |View Full Screen
Geographic Spread of Influenza as Assessed by State and Territorial Epidemiologists:
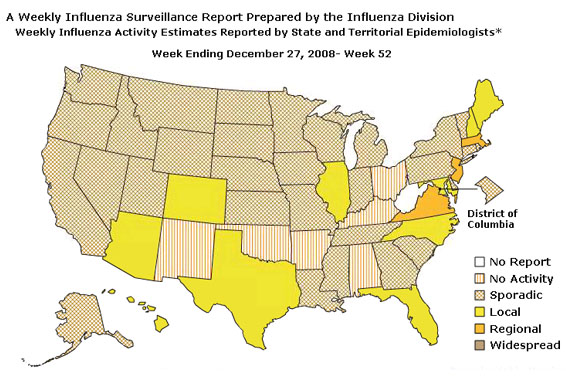
During week 52 the following influenza activity was reported:
- Regional influenza activity was reported by three states (Massachusetts, New Jersey, and Virginia).
- Local influenza activity was reported by 10 states (Arizona, Colorado, Florida, Hawaii, Illinois, Maine, Maryland, New Hampshire, North Carolina, and Texas).
- Sporadic activity was reported in the District of Columbia and 30 states (Alaska, California, Connecticut, Delaware, Georgia, Idaho, Indiana, Iowa, Kansas, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Montana, Nebraska, Nevada, New York, North Dakota, Oregon, Pennsylvania, Rhode Island, South Carolina, South Dakota, Tennessee, Utah, Vermont, Washington, Wisconsin, and Wyoming).
- No influenza activity was reported in six states (Alabama, Arkansas, Kentucky, New Mexico, Ohio, and Oklahoma)
- One state (West Virginia) did not report.
- Content Source: Coordinating Center for Infectious Diseases (CCID)
- National Center for Immunization and Respiratory Diseases (NCIRD)
--------------------------------------------------------------------------------
A description of surveillance methods is available at: http://www.cdc.gov/flu/weekly/fluactivity.htm
- Page last updated January 05, 2008.

