
Table of Contents
FDA Consumer magazine
March-April 2000

by Larry Thompson
The reports of food poisoning trickled in to California public health officials in 1998 but didn't point to an offending product or even show whether these were just sporadic cases or whether an outbreak had begun. In cases where everyone at a local picnic eats the potato salad and gets sick, the culprit is usually pretty clear. But with sporadic cases scattered over time and geography, there are seldom enough clues to track down the cause.
In the California case, however, scientists succeeded in isolating the offending microorganism from the stools of infected people. They found Shigella, a particularly nasty germ that causes gripping abdominal pain, diarrhea and dehydration that can go on for days and even weeks. In young children and the elderly, it can kill. Shigella caused the California outbreak, but where was it coming from?
The key clue came from Minnesota, where State Health Department scientists identified two more Shigella outbreaks and notified U.S. and Canadian public health officials. This allowed federal scientists to compare the California and Minnesota isolates using a relatively new laboratory procedure called pulsed-field gel electrophoresis (PFGE) that gives a unique genetic fingerprint of an organism. PFGE showed that two of the outbreaks were caused by the same strain of Shigella.
"The DNA fingerprint shared by these isolates was different from most of the other Shigella we had seen," says Farukh M. Khambaty, Ph.D., a microbiologist in the Food and Drug Administration's Center for Food Safety and Applied Nutrition. "This suggested that more scrutiny and possible regulatory intervention was needed to identify and rectify the source of the pathogen to prevent further cases or a potentially larger outbreak."
Investigators in the different states compared those who got sick with those who did not get sick to find the offending foods. Raw curly parsley became a prime suspect. By now, there were six outbreaks of foodborne illnesses, including cases in Massachusetts and Canada, as well as Minnesota and California. Public health investigators from the local, state and even provincial health departments in Canada and investigators from FDA and the national Centers for Disease Control and Prevention collaborated in tracking the contaminated food to a parsley producer in Mexico. Using its regulatory authority, FDA blocked imports of any more parsley from that producer until the contamination was eliminated.
"If we had not discovered the source of the problem," Khambaty says, "that [Mexican] operation might have continued unchanged and we could have had a continuous stream of sporadic cases."
The outbreak ended because FDA experts and their other public health colleagues now use an advanced scientific tool to "fingerprint" the microorganisms involved, even though it was originally developed for research in molecular biology labs.
It is just one example of the ways FDA uses science to defend the nation's health.
Future Needs, Immediate Concerns
Science and law. FDA remains one of those rare institutions that relies on both. And while statutes such as the federal Food, Drug, and Cosmetic Act give FDA the authority to regulate various industries and products, it is the science that provides the knowledge needed to develop and apply the regulations in the right way.
"Science is the cornerstone of good regulatory decisions," says FDA Commissioner Jane E. Henney, M.D. "A high-performing, science-based agency like FDA reaps public health benefits for individual citizens and the nation as a whole."
Science at FDA is wide-ranging. It is not just the solitary investigator at the laboratory bench doing original research, though there are some of those. And it is not just the physician asking questions about the safety and effectiveness of new therapeutics, though there are plenty of those. Nor is it only the inspector using scientific techniques to assess the safety and freshness of fish, imported produce or food served on planes, trains and ships, though there are some of those, too.
Science at FDA is all of those things and more. It encompasses the totality of technical knowledge regarding foods and medical treatments. The agency's use of science covers disciplines as diverse as molecular biology and clinical pharmacology, nuclear physics and electrical engineering, clinical trials methodology, and the arcana of statistical analysis of surveillance databases. Given the breadth of the scientific information used by the agency, FDA's scientists and managers must work to remain current lest they miss something and put the country's public health in jeopardy.
"FDA scientists must know the universe of scientific knowledge in their field to the best of their ability," says Margaret Porter, FDA's chief counsel. "You have to try to get your arms around the whole body of information because there are legal consequences to the judgments you make in the way an ivory tower researcher never has to worry about."
FDA's science exists as a complex duality. It's both basic and applied--that is, sometimes creating new knowledge, at other times using existing information to solve practical problems. It is collaborative and collegial but also evaluative and decisive in its regulatory responsibility. FDA's scientists wrestle with cutting-edge concepts in a cosmos of constantly changing scientific advancement yet must come to practical, applied conclusions about complicated products that manufacturers want the American public to use now. The consequences may be nothing less than life and death.
And all the while, FDA's scientists carry out their work in public, through a transparent process that allows other investigators and critics to look over their shoulders and publicly judge their performance and their conclusions.
"We try to make decisions that are defensible among our peers who understand the same science that we use to make the decision," says FDA's acting Deputy Commissioner and chief scientist Bernard Schwetz, D.V.M., Ph.D. "The decisions have to be credible, not only with our peers but also with the general public, who may not understand the data or the scientific aspects of what it takes to make the decision."
More importantly, from the public's point of view, credibility comes from the agency being right most of the time, never an easy achievement given the increasing complexity of the science and the resulting products. Over the last two decades, the rate at which FDA had to take approved drugs off the market because of unexpected adverse events has held constant, if not actually declined slightly, despite a dramatic rise in the number of new products approved.
"The public trusts the decisions that we make," Schwetz says. "They stand back and say, FDA has approved this product and it knows what it is doing. We don't have to second guess it."
What Is Science?
In the sixth century B.C., the Greek natural philosopher (read: scientist) Thales of Miletus started an argument that gave rise to one of science's essential principles: criticism. Thales postulated that water was the basic element of all matter. His own disciple, Anaximander, immediately challenged Thales, pointing out that water is essentially wet and the opposite of wet is dry. Since most things in the world are dry, water could not be the basic element of all matter.
Thales, of course, was wrong, and the debate set the stage for the deliberative process that would forevermore characterize the field of science. Theories and hypotheses. Impressions and interpretations. All must be tested in the crucible of observation, experimentation and argument. An idea or belief unsubstantiated by data will be shoved aside by consensus based on more reliable information.
This principle makes science a rough and tumble free-for-all. It is a way of knowing the workings of the natural world, a system in which questions are asked and then answered with facts derived through specific procedures shown to give reliable results. And a key characteristic is a skepticism that attempts to prevent premature conclusions and generalizations, replacing assumptions with facts.
In the stereotypical view, the white-coated researcher struggles to answer the riddles of the universe. At FDA, the science extends beyond investigator-initiated basic research to include applied studies that serve the needs of the agency's regulatory mission. This includes setting standards for activities as varied as laboratory techniques to determine whether a drug is safe and effective, surveillance for unexpected threats to the public health from most foods and all medical products, and regulatory enforcement that relies on the best analytical tools.
"Our science is very mission oriented, very pragmatic," says Elizabeth Jacobson, Ph.D., deputy director for science in FDA's Center for Devices and Radiological Health. "But mission oriented and pragmatic are not synonymous with dull and uninteresting. Our science is very cutting edge."
And the cutting edge can be a complex place filled with contradictions and dualities. Science is a way of knowing, but its wisdom also illuminates the limits of knowledge. The facts of science provide some certainty--today. Tomorrow, the relentless acquisition of new information may make today's facts incomplete, naive or even wrong. As a result, science is often not black and white, but rather a statement about a range of grays from very black to very white, a set of probabilities from completely true to completely false.
Product Review Science
For FDA, robust scientific data offer the appeal of certainty that new drugs and biologics will be safe and effective, foods won't be dangerous to eat, medical devices will work and not harm the patient, and cosmetics will be safe to use. But the data are never perfect. There is often some degree of uncertainty, and that is a constant source of tension since FDA can only make its regulatory decisions with the best information available at that moment.
"We will never be working in an environment where there is no uncertainty," Schwetz says.
In the face of uncertainty, science has developed a culture of consultation, of peer review. This is a tradition in which experts, peers, sit in judgment of the scientific data and its interpretation. At professional gatherings and in official capacities, scientists will argue over the meaning of a result, the interpretation of a fact. The winning conclusion is based on the strength of the argument, the data, and consensus among those involved in the debate.
In many ways, FDA functions as a super peer review group when it sits in judgment of the scientific data supporting a proposal to market a new drug, biological product, medical device, or food additive. The agency demands the company demonstrate, with scientific evidence, that its product is safe and effective before it may be offered to the public.
"Most drug approval decisions are not that hard," says Robert Temple, M.D., associate director for medical policy in FDA's Center for Drug Evaluation and Research (CDER). "We look at what we do know about the benefits and adverse side effects, ask if there is something critical that we should know that we don't, and then decide. When there is a difficult judgment, we have internal and external consultants and advisory committees available to provide expert opinions."
"The drug development process is a wonderful scientific endeavor," says Roger Williams, CDER's former deputy director for pharmacological science who recently became president of the U.S. Pharmacopoeia. "You start with a molecule that may do something useful clinically, show scientifically that it does so, produce millions of bits of data that all result in what has been called the user's manual for the molecule [product labeling], with the understanding that this scientific data can predict safety and efficacy responses in the general population and in individual patients."
The FDA process is collaborative and collegial, even if the final decision is absolute and regulatory. A team of internal FDA reviewers examines the various scientific elements in a new drug's application to go on the market. And, commonly, the data also will be reviewed by an advisory committee of external experts in the field. When a new therapeutic agent meets the agency's standards for safety and efficacy, when all the questions are answered, the product can go on the market.
For FDA to be effective and credible as a super peer review agency, however, its scientists must remain at the forefront of their fields. "Peer review means review by those at the same level that you are at," says Neil Goldman, Ph.D., associate director for research at FDA's Center for Biologics Evaluation and Research. "So, if you are a manufacturer, and your peers are reviewing you, they [the peer reviewers] need to have the same understanding of the product's mechanism of action or toxicity, therapeutic modalities for testing the product, and all aspects of the manufacturing processes. Only then are they able to help in the evaluation of what you [the manufacturer] propose to do to ensure that the product is safe and effective."
Keeping FDA's scientists current has become a significant concern for the commissioner. "The Food and Drug Administration reviews and makes judgments regarding new products that are the result of cutting-edge science," Henney says. "We must have the capability to keep up with these scientific developments because the judgments we render depend on it."
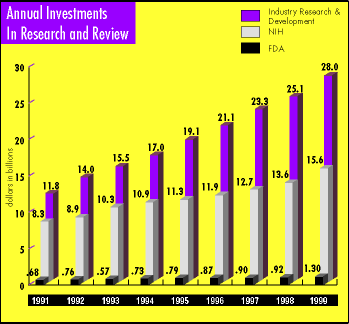
Maintaining that level of scientific expertise has not been easy, say several of FDA's top managers. With declining budgets, FDA strives to devote resources to support its scientific enterprise, to attract and retain highly trained scientists and physicians, and to ensure that the ongoing education of the agency's experts keeps them current in their field of expertise.
And there is no question that new skills and knowledge will be needed. The national investment in basic research has grown exponentially since World War II, with the National Institutes of Health pumping some $16 billion into biomedical research annually and the pharmaceutical and medical device industries adding more than $28 billion in R&D spending last year.
Sometimes, FDA's centers can see the cutting edge coming. Scientists in the Center for Devices and Radiological Health, for example, realized a number of years ago that they would soon be called on to review applications for medical devices that used sophisticated types of artificial intelligence such as "neural networks." Neural networks use very simple processors, all acting in parallel in a network, to process information much like the brain does. Since biological systems are much better than standard computers (which process information in a series) at things like pattern recognition, computer scientists hope to emulate the brain's capacity for pattern recognition by using neural networks. Medical device designers already are exploiting these properties of artificial intelligence to create "smart" devices like automatic Pap smear readers to do repetitive pattern recognition tests. Machines are better than people at these tasks since they don't get tired, or bored, or frustrated.
"Our scientists saw that the use of artificial intelligence in medical devices was on the horizon and that we needed to have expertise in the area. As a result of our investment in this area, when the first application came in the door, we were ready for it," Jacobson recalls. "In fact, our scientists are now leaders in the field: Two of our scientists chaired the International Joint Conference on Neural Networks last year, and one of them is the incoming president of the International Neural Network Society."
"If we do not anticipate the next question, the science would keep moving around us," Schwetz says. "Other scientists are continually generating new information that has to be taken into account."
Moreover, if FDA is not prepared to deal with new science, it could become a bottleneck in the flow of new medical treatments to the patients and health professionals who await them.
"If we [FDA's reviewers] understand the technology and product when it arrives," explains Susan Homire, D.V.M., senior science policy analyst in the Office of the Commissioner, "then we can review it much more efficiently. If it is something we have never seen before, then there is a learning curve that we have to go through and we become conservative. We are afraid to say it is OK. And we ask for more data."
"The ill-prepared are generally risk-averse, therefore giving no decision or a slow decision, or, even worse, the wrong decision," adds Commissioner Henney. "We cannot afford to be a bottleneck in providing physicians, dentists, veterinarians, and other health professionals with safe and effective new products for their patients, but we must also be mindful that these decisions are right."
Science and the Law
The commissioner's commitment to strengthening the agency's scientific foundation has a certain urgency. FDA's science-based decisions must not only protect the public health and pass muster with its scientific peers but challenged decisions must be able to withstand judicial scrutiny as well.
"FDA doesn't do science in the abstract," says FDA's chief attorney Porter. "FDA is a regulatory agency. While we can provide training and set clear expectations for product testing and good manufacturing practices, we are not advisors or consultants; we are regulatory decision-makers."
Certainly FDA's scientists must be decisive when reviewing a new medical product, but tensions sometimes arise between FDA's scientific and legal cultures. "The interface between the legal and science systems doesn't always mesh very well," says scientist Schwetz. "The scientists like to equivocate and not say yes or no. The lawyers only want yes or no answers and they don't want the scientists to hedge."
Each culture--science and law--has its own perspective.
"The scientists' preference is always to keep options open," acknowledges Porter, but she adds, "Think about what the regulated industry needs from the agency. The industry needs predictability. And legally, if you want to hold people to standards, the standards that you use must be clear and predictable and applied consistently."
Out of the dynamic tension between the two cultures frequently come well-honed decisions shaped by a rigorous process of debate. "Decisions work best through mutual recognition of the contribution and the need for each of the disciplines," Porter says, "so that the lawyers understand the needs of the scientists and the scientists understand the needs of the lawyers."
And in the end, concludes the commissioner, everyone within FDA must work to understand the needs of the public and find the best science to protect and promote its health as the agency has done for nearly a century.
Larry Thompson is a member of FDA's public affairs staff.
Table of Contents | How to Subscribe | Back Issues | FDA Home Page
Questions concerning the editorial content of FDA Consumer should be directed to FDA's Office of Public Affairs.
(Hypertext created by clb 2000-FEB-13)