skip
navigation  |
800-411-3222 (Español)
Arsenic in Poultry Litter: Organic Regulations |
| By Barbara C. Bellows NCAT Agriculture Specialist © NCAT 2005 ATTRA Publication #IP266/269 |
The
printable PDF version of the entire document is available
at: http://attra.ncat.org/attra-pub/PDF/arsenic_poultry_litter.pdf 12 pages — 554 KB Download Acrobat Reader |
Abstract
Table of ContentsOverviewMany organic producers use poultry litter—fresh, composted, or as pellets—as a fertilizer and a source of organic matter. Much of this litter comes from commercial broiler operations, which use arsenic as a feed additive to control parasites and increase weight gain. Most of this arsenic does not accumulate in the poultry meat, but is excreted by the birds. As a result, almost 90% of the arsenic fed to poultry ends up in the litter. Because this heavy metal has the potential to build up in the soil, cause health problems for farm workers and produce consumers, and be transported to adjacent lakes or streams, many organic farmers and their certifiers are concerned that using commercial poultry litter is not compliant with the National Organic Standard. Their concerns about organic compliance center on 7 CFR §205.203(c) of the National Organic Program Regulatory Text.(1)
Arsenic also appears on the National List of Prohibited Non-synthetic Substances in §205.602(a). To understand when and how arsenic in poultry litter can contaminate crops, soil, or water, we need to examine the following questions.
What is the source of the poultry litter?
Arsenic, in the forms of roxarsone and arsenilic acid, is an additive in the feed of conventionally-raised broilers. It is used to control protozoan parasites known as coccidians and to enhance weight gain.(2) Feeding arsenic to laying hens is prohibited. Organic regulations prohibit feeding arsenic to birds raised for organic certification. Therefore, you can be sure that you are not using poultry litter containing arsenic by getting it from layer operations or from organic poultry farms. You can also use other methods to enhance the nutrient content of your soil, such as green manure crops, cattle manure, compost, or a mixture of organically-approved inputs, such as fish emulsion, blood meal, and rock phosphate. (Use care when selecting sources of rock phosphate, since it can be contaminated with arsenic, lead, and cadmium.) How much arsenic is in poultry litter?Roxarsone is added to poultry feed at the rate of 22.7 to 45.4 grams per ton, or 0.0025 to 0.005 percent.(3) Most of the roxarsone passes through the birds and is excreted unchanged.(4) Each broiler excretes about 150 milligrams of roxasone during the 42-day growth period in which it is administered.(5) Litter collected following a single flock of birds can contain from 1 to 70 milligrams of arsenic per kilogram of litter, with 30 to 50 milligrams per kilogram commonly found (also expressed as 30 to 50 ppm or 0.003 to 0.005 percent arsenic).(4, 5, 6, 7) Often, poultry houses are only partially cleaned following each flock of birds, increasing the arsenic concentration in the litter.
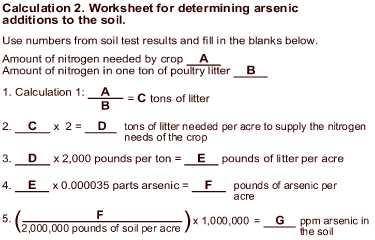
To find out how much arsenic is in the poultry litter that you intend to use, have your soil or manure testing laboratory check your litter samples for arsenic (it may be listed on the soil test request form by its chemical symbol, As). You can ask for this test when you submit your litter for the standard analysis of plant-available nutrients. Depending on the laboratory, the test for arsenic will be labeled either as "arsenic" or as "total recoverable metals," where arsenic is one of several metals analyzed. A soil test for arsenic will range from $5 to $20 per sample, depending on whether you are using a state or private soil testing laboratory. How much arsenic is added to the soil when you apply poultry litter?The amount of arsenic added to the soil depends on how much arsenic is in the litter and how much litter you apply. Calculation 1 provides an example for determining how much arsenic you apply to the soil when you use arsenic-containing litter. Calculation 2 provides a worksheet to determine these amounts for your own fields. To determine the actual amount of arsenic that you are applying with a given load of poultry litter, submit a sample of your soil and the litter to a laboratory for analysis. Then perform the calculations provided below based on crop nutrient needs and using the laboratory values provided for soil nutrients, nutrient content of the litter, and arsenic content of the litter.
How often is poultry litter applied to the same land?Many growers regularly use poultry litter. If you do this with poultry litter containing arsenic, arsenic will build up in the soil. Unlike nutrients that are removed from the soil at predictable rates by crop harvests, heavy metals such as arsenic may or may not be taken up by crops and removed from the field through harvests. You can use the template below to develop a table for monitoring the potential buildup of arsenic in each of your fields. For these records, assume that arsenic accumulates in the soil and is not lost or removed over time.
Only a few states regulate the application of arsenic-contaminated poultry litter, with 23 states having action levels for soils contaminated with arsenic.(8) Maximum acceptable arsenic levels in soils vary widely from state to state. For agricultural soils, action levels range from 2.4 ppm in Arkansas to 500 ppm in Montana, while action levels for residential soils range from 0.4 ppm in Illinois to 40 ppm in Colorado. South Carolina, which has a large poultry industry, has set 41 ppm (dry weight basis) as the maximum concentration of arsenic in poultry litter to be applied to land, with no more than 37 total pounds of arsenic ever to be applied.(9) In contrast, the maximum allowable concentration of arsenic in compost is 10 ppm in Italy, 13 ppm in Canada, 15 ppm in the Netherlands, and 25 ppm in Denmark.(10) Soil arsenic levels of 10 ppm can have a phytotoxic effect (cause plants to die).
A producer applying litter containing 25 ppm of arsenic, at the rate of 5 tons per acre per year, will be able to make similar applications for 3 years before reaching the residential action level in Illinois, for 19 years before reaching the agricultural action level in Arkansas, and 148 years before reaching the maximum arsenic application levels in South Carolina. It would take 80 years of such poultry litter applications to reach the phytotoxic level of 10 ppm. How do soil type and soil management affect the movement and toxicity of arsenic?Chemical and microbial reactions readily transform roxarsone into inorganic forms of arsenic.(13) These inorganic forms are then subject to a variety of chemical and biological reactions in the soil. Soil mineralogy, soil moisture, soil pH, and microbial reactions all determine arsenic mobility, its uptake by plants, and its toxicity. To help you understand these interactions and how they affect the availability of arsenic, the box below provides basic information on soil and arsenic chemistry.
Understanding the basic concepts of arsenic and soil chemistry will help you manage your soil to favor arsenic bonding to soil particles, minimizing its movement and availability to plants. When arsenic is bound to soil minerals, it is relatively immobile. However, it may be transported by erosion. When arsenic is dissolved in water, it can be taken up by plants and is subject to runoff or leaching. Thus, dissolved arsenic is more likely to damage the environment, affect crop growth, or endanger animal and human health than is arsenic that is bound to soil particles. Arsenic is more likely to bind to soil particles in soil that is (14, 15)
Arsenic is more likely to be soluble in soil that is
In wet soils that have a high (alkaline) pH, soil chemistry will favor arsenite over arsenate, resulting in high arsenic toxicity.
In practical terms, if you add poultry litter containing arsenic to upland, arable soils that have loamy or clay-like textures, a neutral or semi-acid pH, and are not subject to waterlogging, the arsenic will be relatively stable in the soil and have a relatively low toxicity. In contrast, if you add poultry litter containing arsenic to soils that are wet, alkaline, or have a sandy texture, the arsenic will have a high toxicity and a high potential for contaminating ground or surface water through leaching and runoff. Plant uptake of arsenic will be greatest on sandy soils with low to moderate levels of organic matter and excessive amounts of phosphorus or nitrate.
The greatest risk of contamination from arsenic in poultry litter comes when litter is removed from the poultry house but not mixed with soil. This happens when the litter is stacked in piles before spreading, or when it is applied to the soil and not mixed in through tillage, rotary cultivation, or the use of an aerator. In the Delmarva Peninsula (adjacent parts of Delaware, Maryland, and Virginia), the area with the second-highest concentration of poultry production in the U.S., research shows that arsenic easily leaches from poultry litter when it is either piled in windrows or has been recently applied to the soil surface. In both cases, arsenic is not able to bind to the soil. Consequently, rainfall readily dissolves the arsenic, making it subject to runoff and leaching.(16, 17) How does increasing soil organic matter affect the potential for arsenic runoff or leaching?
Interactions between soil organic matter, soil minerals, and arsenic are complex. Almost all of the scientific studies of the interactions between arsenic, soil minerals, and soil organic matter have used purified forms of these components.(18, 19) However, agricultural soils are a complex mixture of soil minerals and organic compounds; thus, experiments with pure compounds do not clearly predict what will happen in natural, mixed systems. Recent research, conducted with dissolved organic matter from natural systems in Colorado, indicates that organic compounds tend to displace arsenic bound to iron oxides, resulting in the release of dissolved arsenic into the soil. This process not only increased the amount of dissolved arsenic but also its availability and toxicity, since the organic matter displaced arsenite more readily than arsenate.(20) In another study, kaolinite clay coated with humic acid absorbed more arsenic than did pure kaolinite clay.(21) These results seem to indicate that organic matter will enhance arsenic sorption in temperate soils but will increase arsenic solubility in highly weathered soils. What is the potential that arsenic from applied poultry litter will contaminate rivers, streams, lakes, or groundwater?For arsenic to cause contamination, it must be transported from the field where it was applied to a body of water. To reduce the risk of arsenic runoff, do not apply or store litter containing arsenic within 100 feet of lakes or streams. At least 50 feet of this buffer strip should be in permanent vegetation.(22) To minimize the movement of arsenic into the groundwater, do not apply arsenic-bearing poultry litter to sandy soils with low organic matter content or to soils with underlying karst geology (limestone with caves and sinkholes).
How does arsenic in soil affect crop growth and food safety?Plants differ in their sensitivity to arsenic. Peas and beans are very sensitive to arsenic in the environment, while some species of ferns accumulate large quantities of arsenic without apparent adverse effects.(12, 23, 24) Some plants are arsenic-resistant or can block arsenic uptake by their root systems.(25) Still other food crops accumulate arsenic at levels that raise human health concerns. Since plants take up arsenic primarily by their roots, the highest level of arsenic accumulation is usually in roots and tubers, such as carrots and potatoes. Signs of arsenic phytotoxicity vary among plant species, but these typically include leaves dying back from their tips, stunting, and sterile or abnormal fruits and seeds, similar to phosphorus deficiencies.(14)
Plants react to arsenic at much lower doses than those necessary to have an impact on human health. That is, most plants die before they produce a food product that is toxic when consumed in normal amounts.
Other than poultry litter, what else can contaminate soil with arsenic?The use of commercial broiler litter as a fertilizer or soil amendment is not the only organic input likely to cause arsenic buildup in the soil. Rock phosphate, various igneous rocks applied for "sulfur" and "iron" deficiencies, and waste from copper-chromium arsenate (CCA) treated lumber can also result in high levels of arsenic contamination. In addition, organic standards do not regulate the condition or quality of soils being converted to organic production. Although now illegal, arsenic pesticides were once used on cotton, rice, and fruit. Sewage sludge also often contains arsenic waste products from industry.(26) As mentioned above, the natural background level of arsenic in soils varies considerably—from 0.1 to 97 ppm—with soils derived from igneous parent material tending to have higher natural concentrations. If you are buying land and do not know the history of pesticide or sewage sludge use on the farm, you should have the soil tested to determine the level of arsenic in it. Producers farming soil that contains relatively high levels of arsenic should be particularly mindful when using commercial broiler litter as a fertilizer or soil amendment. How can you remediate arsenic-contaminated soil?If you are managing litter applications carefully, you will probably never need to remediate your soils. As discussed above, there is often little relationship between the total arsenic content of your soil and the amount of arsenic that is bio-available or readily taken up by plants. If you want to know the amount of arsenic taken up by your crop, you can send plant tissue samples to a soil and plant tissue analysis laboratory. For more information on soil remediation, see Arsenic Treatment Technologies for Soil, Waste, and Water, a comprehensive document on arsenic remediation methods developed by the U.S. Environmental Protection Agency.(27) Plants that can take up large quantities of arsenic—called hyperaccumulating plants—are being examined for possible use in the bioremediation of arsenic-contaminated soils.(23, 24) In bioremediation, hyperaccumulating plants are used to extract arsenic from soil to reduce its concentration. Following their harvest, the hyperacculating plants need either to be disposed of as hazardous waste or, preferably, subjected to processes that extract the arsenic from the plant tissue for use by industry. While not hyperaccumulators, poplar trees, cottonwoods, corn, sunflowers, ryegrass, and prairie grasses have been used in phytoremediation projects to remove arsenic from the soil.(27) SummaryArsenic in poultry litter poses a contamination risk to organic crops and soil. At a minimum, organic producers should use practices that are not high risk, according to the following risk assessment table. While the NOP rule is not clear regarding the use of poultry litter containing arsenic as a fertilizer or soil amendment, an accredited certifying agent might require that a farm plan address the potential arsenic contamination of soil and water posed by broiler litter. Such a plan would involve documentation to certify that management practices are not contributing to environmental degradation or health risks.
References1. The National Organic Program. Program Standards. 2. Source, Transport, and Fate of Arsenic in the Pocomoke River, A Poultry Dominated Chesapeake Bay Watershed. 3. Miller, C.V., T.C., Hancock, and J.M. Denver. 2000. Environmental Fate and Transport of Arsenical Feed Amendments for Animal Agriculture. American Geophysical Union, 2000 Spring Meeting: Integrative Geoscience Solutions—A Start for the New Millennium. May 30–June 3. Washington, DC. 4. Kpomblekou, A. K., R. O. Ankumah, and H.A. Ajwa, 2002. Trace and nontrace element contents of broiler litter. Communications in soil science and plant analysis. Vol. 33, No. 11/12. p. 1799–1811. http://taylorandfrancis.metapress.com/openurl.asp?genre=article&id=doi:10.1081/CSS-120004823 5. Garbarino, J.R., D.W. Ruherford, and R.L. Wershaw. No date. Degradation of roxarsone in poultry litter. 6. Sims, J.T., and D.C. Wolf. 1994. Poultry waste management. Agricultural and Environmental Issues. Advances in Agronomy. Vol. 52. p. 1–83. 7. Jackson, B. P., P. M. Bertsch, M. L. Cabrera, J. J. Camberato, J. C. Seaman, and C. W. Wood. 2003. Trace element speciation in poultry litter. Journal of Environmental Quality. Vol. 32. p. 535-540. 8. Baldwin, Linda, and Heather McCreary. No date. Study of state arsenic regulations. Association for the Environmental Health of Soils. Amherst, MA. 9. Chastain, John P., James J. Camberto, and Peter Skewes. 2002. Poultry Manure Production and Nutrient Content. South Carolina
Confined Animal Manure Managers
Certification Program–Poultry. Clemson Extension. Clemson University, Clemson, SC. 10. Brinton, William. 2000. Compost Quality in America. Woods Hole Research Laboratory. Prepared for New York State Association of Recyclers. 11. Shacklette, H.T., and J.G. Boerngen. 1984. Element concentrations in soils and other surficial materials of the conterminous United States. U.S. Geological Survey Professional Paper 1270. United States Government Printing Office. Washington, DC. 12. Fritz, Walter J., and Walter W. Wenzel. 2002. Arsenic transformations in the soil-rhizosphere-plant system: fundamentals and potential application to phytoremediation. Journal of Biotechnology. Vol. 99, Issue 3. p. 259–278. 13. Bednara, J., J. R. Garbarino, I. Ferrera, D. W. Rutherford, R. L. Wershaw, J. F. Ranvillea, and T. R. Wildemana. 2003. Photodegradation of roxarsone in poultry litter leachates. The Science of the Total Environment. Vol. 302, Issues 1-3. p. 237–245. 14. Smith, E., R. Naidu, and A.M. Alston. 1998. Arsenic in the soil environment: A review. Advances in Agronomy. Vol. 64. p. 149–195. 15. Turpeinen, Riina. 2002. Interactions between metals, microbes and plants—Bioremediation of arsenic and lead contaminated soils. Department of Ecological and Environmental Sciences. University of Helsinki. 16. Christen, Kris. 2001. Policy News – March 22, 2001: Chickens, manure, and arsenic. Environmental Science and Technology. Vol. 35, Issue 9. p. 184. 17. Hancock, T.C., J.M. Denver, G.F. Riedel, and C.V. Miller. No date. Reconnaissance for
arsenic in a poultry dominated Chesapeake Bay Watershed—Examination of source,
transport, and fate. U.S. EPA Workshop on Managing Arsenic Risks to the Environment:
Characterization of Waste, Chemistry, and Treatment and Disposal. 18. Grafe, M., M.J.Eick, and P.R. Grossl. 2001. Adsorption of Arsenate (V) and Arsenite (III) on Goethite in the Presence and Absence of Dissolved Organic Carbon. Soil Science Society of America Journal. Vol. 65. p. 1680-1687. 19. Cornu, S., D. Breeze, Alain Saada, and P. Baranger. 2003. The influence of pH, electrolyte type, and surface coating on arsenic (V) adsorption onto kaolinites. Soil Science Society of America Journal. Vol. 67. p. 1127–1132. 20. Redman, Aaron D., Donald L. Macalady, and Dianne Ahmann. 2002. Natural organic matter affects arsenic speciation and sorption onto hematite. Environmental Science and Technology. Vol. 36. p. 2889–2896. 21. Saada, A., D. Breeze, C. Crouzet, S. Cornu, and P. Baranger. 2003. Adsoption of arsenic (V) on kaolinite and on kaolinite-humic acid complexes. Role of humic acid nitrogen groups. Chemosphere. Vol. 58, No. 8. p. 757-763. 22. National Research Council. 2002. Riparian Areas: Functions and Strategies for Management. National Academy Press, Washington, DC. 23. Ma, Lena Q., Kenneth M. Komar, Cong Tu, Weihua Zhang, Yong Cai, and Elizabeth D. Kennelley. 2001. A fern that hyperaccumulates arsenic. A hardy, versatile, fast-growing plant helps to remove arsenic from contaminated soils. Nature. Vol. 409. p. 579. 24. Wang, J., F.J. Zhao, A.A. Meharg, A. Raab, J. Feldmann, and S.P. McGrath. 2002. Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiology. Vol. 130, No. 3. p. 1552–1561. 25. Meharg, Andrew A., and Jeanette Hartley-Whitaker. 2002. Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytologist. Vol. 154. p. 29–43. 26. EPA. 1998. Locating and estimating air emissions from sources of arsenic and arsenic compounds. United States Environmental Protection Agency. Office of Air Quality. Triangle Park, NC. 27. U.S. EPA. 2002. Arsenic Treatment Technologies for Soil and Water. Solid Wastes and Emergency Response. United States Environmental Protection Agency. Acknowledgements: The author would like to express her appreciation to her colleague George Kuepper, Brian Baker from the Organic Materials Review Institute, and Diane Tracy from Antech for their careful review of this publication and insightful comments.
By Barbara C. Bellows
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Site Map | Comments | Disclaimer | Privacy Policy | Webmaster Copyright © NCAT 1997-2009. All Rights Reserved. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||