 Depending on where you live, maybe you've heard of acid rain. Now, acid rain is not pure acid falling from the sky, but rather it is rainfall or atmospheric moisture that has been mixed with elements and gases that have caused the moisture to become more acidic than normal. Pure water has a pH of 7, and, generally, rainfall is somewhat on the acidic side (a bit less than 6). But, acid rain can have a pH of about 5.0-5.5, and can even be in the 4 range in the northeastern United States, where there are a lot of industries and cars.
Depending on where you live, maybe you've heard of acid rain. Now, acid rain is not pure acid falling from the sky, but rather it is rainfall or atmospheric moisture that has been mixed with elements and gases that have caused the moisture to become more acidic than normal. Pure water has a pH of 7, and, generally, rainfall is somewhat on the acidic side (a bit less than 6). But, acid rain can have a pH of about 5.0-5.5, and can even be in the 4 range in the northeastern United States, where there are a lot of industries and cars.
"Acid rain is caused by human activity"
 Acid rain is a uniquely human-related phenomenon. The burning of fossil fuels (coal and oil) by power-production companies and industries releases sulfur into the air that combines with oxygen to form sulfur dioxide (SO2). Exhausts from cars cause the formation of nitrogen oxides in the air. From these gases, airborne sulfuric acid (H2SO4) and nitric acid (HNO3) can be formed and be dissolved in the water vapor in the air. Although acid-rain gases may originate in urban areas, they are often carried for hundreds of miles in the atmosphere by winds into rural areas. That is why forests and lakes in the countryside can be harmed by acid rain that originates in cities.
Acid rain is a uniquely human-related phenomenon. The burning of fossil fuels (coal and oil) by power-production companies and industries releases sulfur into the air that combines with oxygen to form sulfur dioxide (SO2). Exhausts from cars cause the formation of nitrogen oxides in the air. From these gases, airborne sulfuric acid (H2SO4) and nitric acid (HNO3) can be formed and be dissolved in the water vapor in the air. Although acid-rain gases may originate in urban areas, they are often carried for hundreds of miles in the atmosphere by winds into rural areas. That is why forests and lakes in the countryside can be harmed by acid rain that originates in cities.
 The environment can generally adapt to a certain amount of acid rain. Often soil is slightly basic (due to naturally occurring limestone, which has a pH of greater than 7). Because bases counteract acids, these soils tend to balance out some of the acid rain's acidity. But in areas, such as some of the Rocky Mountains and parts of the northwestern and southeastern United States, where limestone does not naturally occur in the soil, acid rain can harm the environment.
The environment can generally adapt to a certain amount of acid rain. Often soil is slightly basic (due to naturally occurring limestone, which has a pH of greater than 7). Because bases counteract acids, these soils tend to balance out some of the acid rain's acidity. But in areas, such as some of the Rocky Mountains and parts of the northwestern and southeastern United States, where limestone does not naturally occur in the soil, acid rain can harm the environment.
Some fish and animals, such as frogs, have a hard time adapting to and reproducing in an acidic environment. Many plants, such as evergreen trees, are damaged by acid rain and acid fog. I've seen some of the acid-rain damage to the evergreen forests in the Black Forest of Germany. Much of the Black Forest was indeed black because so much of the green pine needles had been destroyed, leaving only the black trunks and limbs! You also might notice how acid rain has eaten away the stone in some cities' buildings and stone artwork.
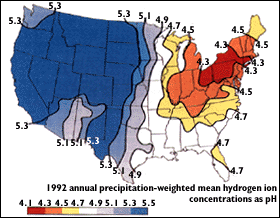 Acidity in rain is measured by collecting samples of rain and measuring its pH. To find the distribution of rain acidity, weather conditions are monitored and rain samples are collected at sites all over the country. The areas of greatest acidity (lowest pH values) are located in the Northeastern United States. This pattern of high acidity is caused by the large number of cities, the dense population, and the concentration of power and industrial plants in the Northeast. In addition, the prevailing wind direction brings storms and pollution to the Northeast from the Midwest, and dust from the soil and rocks in the Northeastern United States is less likely to neutralize acidity in the rain.
Acidity in rain is measured by collecting samples of rain and measuring its pH. To find the distribution of rain acidity, weather conditions are monitored and rain samples are collected at sites all over the country. The areas of greatest acidity (lowest pH values) are located in the Northeastern United States. This pattern of high acidity is caused by the large number of cities, the dense population, and the concentration of power and industrial plants in the Northeast. In addition, the prevailing wind direction brings storms and pollution to the Northeast from the Midwest, and dust from the soil and rocks in the Northeastern United States is less likely to neutralize acidity in the rain.
 When you hear or read in the media about the effects of acid rain, you are usually told about the lakes, fish, and trees in New England and Canada. However, we are becoming aware of an additional concern: many of our historic buildings and monuments are located in the areas of highest acidity. In Europe, where buildings are much older and pollution levels have been ten times greater than in the United States, there is a growing awareness that pollution and acid rain are accelerating the deterioration of buildings and monuments.
When you hear or read in the media about the effects of acid rain, you are usually told about the lakes, fish, and trees in New England and Canada. However, we are becoming aware of an additional concern: many of our historic buildings and monuments are located in the areas of highest acidity. In Europe, where buildings are much older and pollution levels have been ten times greater than in the United States, there is a growing awareness that pollution and acid rain are accelerating the deterioration of buildings and monuments.
Stone weathers (deteriorates) as part of the normal geologic cycle through natural chemical, physical, and biological processes when it is exposed to the environment. This weathering process, over hundreds of millions of years, turned the Appalachian Mountains from towering peaks as high as the Rockies to the rounded knobs we see today. Our concern is that air pollution, particularly in urban areas, may be accelerating the normal, natural rate of stone deterioration, so that we may prematurely lose buildings and sculptures of historic or cultural value.
![]() By the way, no, you don't need to start wearing a rainhat.
By the way, no, you don't need to start wearing a rainhat.
♦
USGS's acid rain Web site
♦ Activities to Explore Acid Rain and Building Stones: USGS Open File Report 95-566
♦ Acidic Deposition ("Acid Rain"): USGS National Biological Service