Final Report: Photo Induced Reduction of Mercury in Lakes, Wetlands, and Soils
EPA Grant Number: R827632Title: Photo Induced Reduction of Mercury in Lakes, Wetlands, and Soils
Investigators: Nriagu, Jerome O. , Keeler, Gerald J. , Lehrnan, John , Lindberg, Steve , Qang, Xia-Qin , Zhang, Hong
Institution: University of Michigan - Ann Arbor , Oak Ridge National Laboratory
EPA Project Officer: Stelz, Bill
Project Period: September 1, 1999 through August 31, 2002 (Extended to September 30, 2003)
Project Amount: $865,771
RFA: Mercury: Transport and Fate through a Watershed (1999)
Research Category: Mercury , Water and Watersheds
Description:
Objective:This objective of this research project was to evaluate the role of photochemistry on the cycling of mercury in the Saginaw Bay watershed. Focus of the investigations included photo-dependent biotic, abiotic, physical, or chemical processes although the reaction mechanisms of interest may be homogeneous or heterogeneous. The transect from Saginaw Bay to the Lake Huron system has well-defined gradients in chemical parameters (including chlorophyll-a, dissolved organic carbon (DOC), phosphorus, nitrogen, chloride, and suspended particulates), trophic conditions, taxonomic composition, and biomass and provides a unique site for the study of the effects of chemical gradients and biological processes on the production of elemental mercury (Hg) in freshwater ecosystems. We measured the ambient airborne Hg levels and fluxes of Hg at many sites covering forest soils, agricultural soils, wetlands, city park soils, beach sands, and industrial/municipal waste dumps. We also measured the Hg concentrations in soil samples from the stations and in water samples from the rivers and various parts of the Saginaw Bay. From the survey data, we selected a number of sites which were monitored intensively over a period of time.
Summary/Accomplishments (Outputs/Outcomes):Ambient atmospheric concentrations of mercury in various parts of the bay varied from less than 1.0 ng/m3 to greater than 10 ng/m3, and the fluxes of Hg from the sites ranged from approximately 1.2 ug/m2 · hr) to more than12 ng/m2 · hr. Concentrations in soils varied from 11 ng/g to more than 1,200 ng/g found at a metal recovery plant site. Significant enrichment (up to 10-fold) of Hg in the forest litter compared to the soil was observed.
Total dissolved Hg concentrations in the surface waters of Saginaw Bay were generally low, in the range of less than 0.2 to 0.9 ng/L. These values are much less than what one would expect in a contaminated aquatic environment classified as an “area of concern.” There were significant spatial differences in dissolved gaseous mercury (DGM) concentrations in surface waters of Saginaw Bay (Table 1). For instance, between June 18 and 19, 2002, observed DGM concentrations at different surface stations varied from 4.7 pg/L to 26 pg/L. On any given day, the DGM levels in surface waters also could vary widely, as exemplified by 23-50 pg/L observed on June 20, 2003.
Table 1. Spatial and Temporal Variations in DGM (pg/L) in Surface Water at Stations in the Inner Saginaw Bay
Station |
Time |
Temp |
DGM* |
Time |
Temp |
DGM* |
Time |
Temp |
DGM* |
|
6/12/01 |
6/12/01 |
6/12/01 |
6/18/02 |
6/18/02 |
6/18/02 |
6/26/03 |
6/26/03 |
6/26/03 |
SB-1 |
xxx |
xxx |
xxx |
1440 |
18.9 |
10.9 |
946 |
xxx |
32.6 |
SB-3 |
xxx |
xxx |
xxx |
1416 |
18.7 |
11.7 |
1052 |
xxx |
22.8 |
SB-4 |
xxx |
xxx |
xxx |
1340 |
18.1 |
5.7 |
1137 |
xxx |
23.9 |
SB-5 |
xxx |
xxx |
xxx |
1205 |
17.5 |
4.9 |
1213 |
xxx |
49.5 |
SB-6 |
1250 |
18.4 |
30 |
1135 |
17.7 |
4.7 |
1244 |
xxx |
26.2 |
SB-7 |
1205 |
18.4 |
25 |
xxx |
xxx |
xxx |
1344 |
xxx |
24.1 |
SB-8 |
1145 |
18.4 |
37 |
1045 |
16.3 |
20.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8/6/01 |
8/6/01 |
8/6/01 |
|
|
|
|
|
|
SBU-1 |
1,200 |
22 |
29.2 |
|
|
|
|
|
|
SBU-2 |
1,328 |
23.5 |
21.6 |
|
|
|
|
|
|
SBU-3 |
1,430 |
23.6 |
21.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8/7/01 |
8/7/01 |
8/7/01 |
6/19/02 |
6/19/02 |
6/19/02 |
6/25/03 |
6/25/03 |
6/25/03 |
SBL-1 |
xxx |
xxx |
xxx |
850 |
18.2 |
4.9 |
1034 |
xxx |
33.4 |
SBL-2 |
xxx |
xxx |
xxx |
1149 |
18.7 |
6.1 |
1157 |
xxx |
28.9 |
SBL-3 |
1200 |
26.1 |
11.7 |
1126 |
18.6 |
8.4 |
1237 |
xxx |
27.2 |
SBL-4 |
1106 |
25.4 |
11.8 |
1105 |
18.4 |
21 |
1304 |
xxx |
24.8 |
SBL-5 |
1038 |
26.1 |
17.2 |
1044 |
18.7 |
22.6 |
1327 |
xxx |
23.5 |
SBL-6 |
937 |
26 |
14.5 |
1019 |
18.3 |
26.5 |
1354 |
xxx |
19.8 |
|
|
|
|
|
|
|
|
|
|
* xxx = no measurements |
|||||||||
Strong diel changes in concentrations of DGM were observed in surface nearshore waters at all of the stations investigated (Figure 1). The DGM concentration could reach approximately 70 pg/L around noontime but declined to only approximately 4 pg/L before sunrise and around sunset at some stations. Our study documented significant spatial and temporal variations in DGM levels in surface waters of the bay, which were strongly dependent on the intensity of sunlight. Besides the marked diel pattern, which peaked around noontime, the DGM concentrations in the nearshore waters of Saginaw Bay at Tawas Bay also exhibited seasonal variations, with the levels in June generally higher than in August. The diel and seasonal variations in DGM concentrations in Saginaw Bay seem to indicate that the dissolved Hg pool, though small, is being recycled actively in the water column. Calculations using reported Henry’s Law constants show that observed DGM concentrations in most water samples (from both the bay and rivers) exceeded expected equilibrium values, suggesting the bay waters generally were oversaturated with Hg relative to the partial pressure of Hg in the atmosphere.

Figure 1. Diel Changes in DGM Typical at Several Stations in Saginaw Bay
Many farms, especially in the eastern parts of the bay, used to be sowed with corn dressed with mercury compounds, and contamination of soils by this pathway was of interest and concern. We investigated the release of gaseous mercury from soils of a Clarksville farm known to have used Hg-dressed seeds. The outflux of Hg from soils varied from less than 0.1 ng/m2 · hr to approximately 1.7 ng/m2 · hr (Figure 2). The low outgasing of Hg suggests that either contamination of soils with mercury from this source was minor or any of the Hg that was introduced by the practice since had been lost. It is remarkable that the fluxes of Hg measured above soybean plants (up to 3 ng/m2 · hr) were higher than the emission rate over the soil alone (Figure 2), suggesting that plants and vegetation are contributing to the release of Hg from soils to the atmosphere within the basin.
In a short-term field study conducted in Tobico marsh, a lagoon wetland at the coast of Saginaw Bay in Bay City of Michigan, very low Hg evasion fluxes (~1-2 ng/m2 · hr) were observed even in strong sunlight. Very low DGM concentrations (~1-4 pg/L) also were found there, implying that the level of Hg in the marsh water was controlled by evasional loss to the atmosphere. Assuming average water depth of 1.0 m, the lifetime of DGM in the marsh was estimated to be about 1 hour.
The measurements both in field and the laboratory lead us to conclude that little of the mercury that has been deposited in Saginaw Bay is retained in the basin. This raises the question as to what is the ultimate sink for much of the mercury that is released in North America.

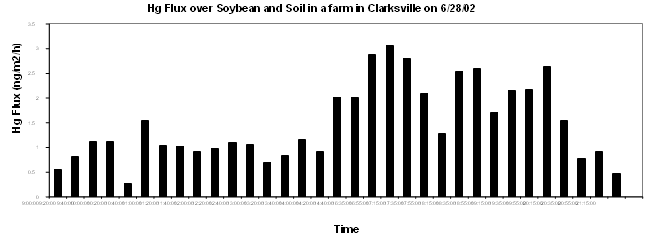
Figure 2. Out-gassing of Mercury From Farm Soils in Saginaw Bay
Journal Articles on this Report: 1 Displayed | Download in RIS Format
| Other project views: | All 4 publications | 3 publications in selected types | All 2 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Zhang H, Lindberg S, Gustin M, Xu X. Toward a Better Understanding of Mercury Emissions from Soils. ACS Symposium Series 2002;(835):246-261. |
R827632 (Final) |
not available |
Saginaw Bay, environmental chemistry, dissolved gaseous mercury, watershed, biogeochemical cycling, air/water exchange, photochemical reactions, soils, wetland, Midwest, heavy metals,
,
Ecosystem Protection/Environmental Exposure & Risk, Water, Air, Geographic Area, Scientific Discipline, Waste, RFA, Ecosystem/Assessment/Indicators, Great Lakes, Ecology, Mercury, Nutrients, Chemistry, Ecological Indicators, Fate & Transport, Hydrology, particulate matter, Ecological Effects - Environmental Exposure & Risk, Ecosystem Protection, bioavailability, heavy metals, lakes, soils, aquatic ecosystem, lake ecosystem, re-emission, hydrological stability, photo induced reduction of mercury, aquatic, fate and transport, anthropogenic disturbances, Lake Huron, methylation, photo induced reduction, lake ecosystems, wetland, wetlands, suspended particulates, ecological exposure, mercury in lakes, aquatic ecosystems, mercury loading, ecosystem, nutrient supply, soil, anthropogenic stress, ecosystem stress
Relevant Websites:
Progress and Final Reports:
2000 Progress Report
2001 Progress Report
Original Abstract
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20081107105040im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)