2002 Progress Report: Center for Air Toxic Metals (CATM)
EPA Grant Number: R827649Center: Center for Air Toxic Metals® (CATM®)
Center Director: Groenewold, Gerald
Title: Center for Air Toxic Metals (CATM)
Investigators: Groenewold, Gerald , Behr-Andres, Christina B. , Benson, Steven A. , Galbreath, Kevin C. , Hassett, J. , Holmes, Michael J. , Jensen, Robert R. , Laudal, Dennis L. , Miller, Stanley J. , Olson, Edwin S. , Pavlish, John H. , Ralston, Nicholas V.C. , Zygarlicke, Christopher J.
Current Investigators: Pavlish, John H. , Benson, Steven A. , Galbreath, Kevin C. , Holmes, Michael J. , Miller, Stanley J. , Zygarlicke, Christopher J.
Institution: University of North Dakota
EPA Project Officer: Stelz, Bill
Project Period: October 15, 1999 through October 14, 2003
Project Period Covered by this Report: October 15, 2001 through October 14, 2002
Project Amount: $4,772,400
RFA: Center for Air Toxic Metals (CATM) (1998)
Research Category: Targeted Research
Description:
Objective:The overall objective of the research project, conducted by the Center for Air Toxic Metals (CATMSM), is to address air toxic trace element emissions that have become a matter of worldwide concern as well as a regulatory issue in the United States. The objective of CATMSM is to develop key information on air toxic metal compounds to support the development and implementation of pollution prevention and control strategies that will effectively reduce air toxic metal emissions and releases to the environment.
Progress Summary:CATMSM research activities this year addressed several key issues related to air toxics. A summary of these research activities and accomplishments is provided below.
Coal Combustion Flue Gas Effects on Mercury Speciation. Program Area
1 of CATMSM has been supporting research in
coal combustion flue gas effects on mercury speciation. It was verified both
by an online mercury analyzer system and by Ontario Hydro mercury sampling and
speciation that 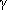 -Fe2O3
reactively captures elemental mercury (Hg0). Combustion testing in a 42-MJ/hour
combustion system revealed that an abundance of Hg2+, HCl, and
-Fe2O3
reactively captures elemental mercury (Hg0). Combustion testing in a 42-MJ/hour
combustion system revealed that an abundance of Hg2+, HCl, and 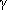 -Fe2O3
in Blacksville coal combustion flue gas catalyzes Hg2+ formation, and that HCl
may be an important Hg0 reactant. In addition, experiments confirmed that the
filtration of Absaloka and Falkirk combustion flue gases through a 150°C fabric
filter (FF) coated with
-Fe2O3
in Blacksville coal combustion flue gas catalyzes Hg2+ formation, and that HCl
may be an important Hg0 reactant. In addition, experiments confirmed that the
filtration of Absaloka and Falkirk combustion flue gases through a 150°C fabric
filter (FF) coated with 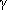 -Fe2O3
results in about 30 percent of the Hg0 in Absaloka and Falkirk flue gases being
converted to Hg2+ and/or Hg(p). One hundred ppmv HCl injection into the Absaloka
combustion flue gas converted most of the Hg0 to Hg2+(gas), whereas HCl injection
into the Falkirk flue gas converted most of the Hg0 and Hg2+ to Hg(p). Addition
of
-Fe2O3
results in about 30 percent of the Hg0 in Absaloka and Falkirk flue gases being
converted to Hg2+ and/or Hg(p). One hundred ppmv HCl injection into the Absaloka
combustion flue gas converted most of the Hg0 to Hg2+(gas), whereas HCl injection
into the Falkirk flue gas converted most of the Hg0 and Hg2+ to Hg(p). Addition
of 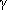 -Fe2O3
and HCl did not have a synergistic effect on Hg0 oxidation. Much heavier FF
loadings of
-Fe2O3
and HCl did not have a synergistic effect on Hg0 oxidation. Much heavier FF
loadings of 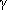 -Fe2O3
essentially doubled the baghouse Hg(tot) removal efficiency. Evidence is insufficient
to decipher whether
-Fe2O3
essentially doubled the baghouse Hg(tot) removal efficiency. Evidence is insufficient
to decipher whether 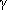 -Fe2O3
reactively captured Hg or if the additional surface area alone provided by heavy
FF loadings of
-Fe2O3
reactively captured Hg or if the additional surface area alone provided by heavy
FF loadings of 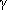 -Fe2O3
accounted for the improved baghouse Hg(tot) capture. In summary, by injecting
economical concentrations of
-Fe2O3
accounted for the improved baghouse Hg(tot) capture. In summary, by injecting
economical concentrations of 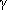 -Fe2O3
as an oxidizing agent upstream of a filtration device, removal efficiencies
for total mercury were doubled.
-Fe2O3
as an oxidizing agent upstream of a filtration device, removal efficiencies
for total mercury were doubled.
In addition to studying oxidizing agents for Hg0, the construction of a portable bench-scale entrained-flow reactor was completed and used to study the transformation rate (kinetics) of Hg0 to Hg2+ and/or Hg(p) in coal combustion flue gas. The reactor was specially designed and constructed for time-temperature studies. Experiments were run using a subbituminous coal that produces a low-acid flue gas. Hg transformations were not detected in the reactor above 275°C, but at 150°C, Hg(tot), and Hg0 were transformed primarily into Hg(p) with increasing residence time.
Development of Sampling and Analytical Tools To Assess Mercury Speciation. Advances in definitive speciation of Hg2+ compounds in a flue gas stream from the development of methods for cryogenic and solvent trapping of certain volatile Hg2+ and their analysis by mass spectrometry (MS) continued. The analysis of mercuric chloride and mercuric nitrate by gas chromatography (GC) and identification by MS, now is better understood, and the unusual behavior of mercuric nitrate on this technique was documented in a refereed publication. A method for transferring mercuric chloride from the initial cryotrap to the GC-MS was developed further, but problems were encountered in the application with high-moisture flue gas. Improvements were made in the construction and use of a continuous vapor source for mercuric nitrate. These insights in the behavior of the oxidized mercury species have benefited the development of a model for the chemisorption of Hg0 on a carbon-sorbent surface. The combination of these efforts and subsequent improvement will lead to quantitative speciation methods with high levels of confidence for the major volatile oxidized mercury species.
Airborne Mercury-Sampling Method Development. The Energy and Environmental Resource Center (EERC), in conjunction with the University of North Dakota (UND) High-Altitude Balloon Group, completed five test-launch missions from August 2001 to December 2002. The goal of this research project is to determine the potential for use of high-altitude weather balloons for sampling atmospheric Hg to determine atmospheric distribution at altitude, downgradient from Hg emission sources.
The payload for the missions included a five-port sampler, gold-coated sand traps, and a 12-L pump. The mission durations were less than 3 hours, and the maximum altitude sampled was 75,400 feet. Results show the distribution of atmospheric mercury concentrations, which range from 0.03 to 1.4 ng/m3. Concentrations are highest nearer the ground surface and decrease with altitude to less than 0.2 ng/m3. Higher concentrations generally are observed below approximately 20,000 feet.
Development of Mercury Control Technologies. This research project focuses on developing and testing promising sorbents and catalysts that have high reactivities to provide rapid in-duct mercury capture or oxidation when injected upstream of a particulate control device. In-duct capture sorbents will provide a useful method for both electrostatic precipitators and baghouses, and the ability to oxidize mercury will make scrubbers more effective in removing mercury.
A dual functionality model for mercury-flue gas interactions with carbon sorbents has been developed. This model shows a two-step oxidation and capture process. Certain acid gases are needed for the oxidation step. As mercury is captured, SO2 poisons the binding site for Hg(II) on the sorbent surface and leads to breakthrough. The mercury emitted from the sorbent after breakthrough is an entirely oxidized mercury species, consistent with the model.
Tests were completed with an entrained-flow reactor to evaluate the effect of residence time on the level of oxidation of mercury when coal is burned in the EERC pilot-scale combustion test facility. Results showed that both lower temperature and longer residence time lead to a higher fraction of oxidized mercury. Entrained-flow reactor tests also demonstrated the effect of residence time and carbon injection rate on in-flight capture of mercury when activated carbon is injected into the flue gas stream.
The EERC-fixed thin-bed reactor has been used for several years as an approach to evaluate a variety of sorbents. Little testing of this system with real flue gas had previously been completed. Direct comparison tests were completed that sampled real and simulated flue gas. The simulated flue gas results were similar to the real flue gas results, and provided confidence that the fixed thin-bed test protocol is a valid approach to screen and compare sorbents.
Modeling and Database Development. The CATMSM modeling efforts have continued to develop approaches to predict and understand toxic trace elements from thermal sources. These efforts include the development and improvement of computer-based predictive tools using statistical and artificial intelligence-based approaches.
The extensive collection of data from governmental, industrial, and research sources was utilized to develop advanced statistical relationships using ash chemistry along with an artificial intelligence-based application to understand the speciation of trace elements produced from coal-fired systems. This information is important for understanding the forms of the trace elements produced and improving the control of the toxic trace elements from thermal sources. The compiled data, along with the computer-based tools, are available to researchers and others interested in trace element information.
Stability of Mercury in Coal Combustion By-Products (CCBs) and Sorbents. This continuing research project focused on three primary tasks, with the goal of determining the mechanisms of mercury release from coal combustion byproducts (CCBs). The tasks included studying the impact of microbial activity on the release of mercury from fly ash, determining the leachability of mercury from various CCBs, determining the level of mercury vapor offgassing from CCBs, and continuing to develop thermal desorption techniques to identify mercury compounds present on CCBs.
The effect of microbial activity on the release of mercury from one fly ash was tested. Hg0 release was measured, but experimental difficulties prevented organomercury measurements. A second fly ash will be evaluated, and the results will be available in the first quarter of 2003.
Leaching was performed on 47 CCB samples using three leaching procedures; both were short- and long-term tests. Two-thirds of the results show no detectable mercury leachability. Leaching will continue as samples are available.
Six CCB samples previously tested through CATMSM were evaluated in duplicate for the release of mercury vapor at ambient temperature. Blank measurements currently are being tested. The improved apparatus used in the current experimentation indicates that the release of mercury vapor is an order of magnitude lower than previously thought. Preliminary results indicate an average release of 2.9 × 10-9 lb Hg/ton CCB/yr.
Thermal desorption curves have been generated for various CCB samples. Experimental work was done to determine the thermal curve for devolatilization of mercuric chloride from quartz granules.
Mercury Releases From Crude Oil and Other Fuels. Information related to the content and fate of mercury from crude oil and other fuels (referred to as alternative fuels) is limited. Evaluation of existing information and additional acquisition and analysis of samples are needed to better quantify the mercury associated with utilization of both crude oils and alternative fuels such as biomass, oil sands, tar sands, and oil shale.
The United States consumes 19.6 MM barrels/day of oil, which is roughly equivalent to the amount of coal consumed domestically on a tonnage basis. Consequently, depending on the associated levels of mercury released to the environment during production and utilization of that oil, the potential exists for significant contribution to the global mercury pool. Currently available literature indicates that mercury concentrations of produced crude oils vary widely from below analytical detection limits to ppm levels, with a majority of the reported samples in the single-digit ppb levels. In comparison, the information collection request (ICR) for coal showed average mercury concentrations for the major coal types ranging from 49 to 126 ppb. Although most of the crude oil samples are at concentrations below these levels, the literature does indicate that there are regions with very high concentrations of mercury (thousands of ppb). Additional research is needed in this area to evaluate mercury concentrations in crude oils at the source, before it is removed, during production, and during processing.
Mercury data for alternative fuels are limited. The need for better data becomes increasingly important as more interest and emphasis is placed on utilizing local and renewable sources of energy such as biomass, oil sands, tar sands, oil shale, etc. In general, mercury levels in sources of biomass are expected to be relatively low, but higher values have been observed (e.g., up to 71 ppb for leaf and tree needle litter). Therefore, more data are needed to identify typical and range values. Limited data show that there also can be significant levels of mercury in oil sands and oil shale and that analysis of a representative sample set is warranted.
Nickel, Chromium, and Arsenic Speciation of Ambient Particulate Matter (PM) in the Vicinity of an Oil-Fired Utility Boiler. Ambient particulate matter (PM), PM10, and PM2.5 were continuously sampled during August 26-31, 2002, from an urban State Air Monitoring Station and a Local Air Monitoring Station site in the vicinity of two oil- and gas-burning power plants. Urban PM, PM10, and PM2.5 samples were analyzed using x-ray absorption fine structure spectroscopy (XAFS) during October 18-22, 2002, at the National Synchrotron Light Source at Brookhaven National Laboratory, New York. XAFS is being used to directly determine the speciation of As, Cr, and Ni in ambient PM, PM10, and PM2.5 samples. Knowledge of the relationship between oil- and gas-burning power system emissions and ambient air quality will ultimately result in a more realistic inhalation-based risk assessment. Such an assessment is necessary for the U.S. Environmental Protection Agency (EPA) to properly address public health risks and regulatory decisions.
Transition Metal Speciation of Fossil Fuel Combustion Flue Gases. Five representative Powder River Basin (PRB) subbituminous coal fly ash samples, containing 45 to 80 ppm Cr, were obtained from the EERC Coal Ash Resources Research ConsortiumSM and analyzed using XAFS. The XAFS data are being reduced and interpreted. Thermochemical equilibrium calculations suggest that carcinogenic hexavalent chromium compounds do not occur in PRB subbituminous coal fly ashes. Results will be available next year.
Fundamental Study of Selective Catalytic Reduction (SCR) Impact on Mercury Speciation. Previous testing conducted by the EERC to evaluate the impact of selective catalytic reduction (SCR) on mercury speciation included 4 weeks of pilot-scale testing and full-scale sampling at six different power plants. The results from these studies indicated that the impact is coal specific. It is speculated that SO2/SO3 and HCl/Cl2 concentrations play a pivotal role. Bench-scale tests using a fixed-bed system will be conducted to help determine the effects of these gases. A full-factorial design is being used to evaluate the independent variables, which include the reactor (none, SCR), presence of acid gases (HCl and SO2/SO3), fly ash type, and residence time. Results will be available next year.
Long-Term Mercury Monitoring. Long-term monitoring (25-30 days) of mercury emissions and data evaluation were completed at two different power plants. The results indicate that mercury emissions can be quite variable, presenting a control challenge. The variability is due to mercury variability in coal, load, changes in operating conditions, and other factors not fully understood.
Mercury Monitoring at North Dakota Power Plants. Previous testing of coal-fired utilities indicates that mercury speciation and emissions are coal-specific. Long-term tests (approximately 20 days) using continuous mercury monitors are needed to investigate variability, especially pertaining to the combustion of lignite coals. Although the testing was on North Dakota utilities, the data are useful beyond the state, because U.S. power plants burning lignite have shown higher Hg0 emissions than plants burning bituminous coals. This form of mercury is more difficult to remove than oxidized forms, and requires innovative measures to control the emissions from the range of combustion and environmental control systems in North Dakota. This research project will provide information that can be used to more accurately estimate mercury emissions from lignite-fired power plants and to begin formulating a comprehensive strategy that will address critical issues related to mercury control. Results will be available next year.
Potential Impact of Selenium on Mercury Toxicity. It has become clear through CATMSM research that the relationship between selenium and mercury is not simply that selenium has a protective effect against mercury toxicity through its ability to bind up the mercury. Instead, mercury toxicity is the result of its selenium sequestration, inducing a deficiency of low molecular-weight selenium necessary for synthesis of the essential selenoenzymes. The lack of these enzymes is particularly dangerous in neuronal tissues, because these cells have no backup systems for free radical detoxification in brain cells. Loss of selenium-dependent free radical detoxification enzymes causes extensive cell damage and death in fetal brains when maternal methylmercury consumption is excessive relative to selenium. Furthermore, once the redox state inside the cell switches from its normal reducing state to become an oxidizing environment, the essential reduced forms of selenomolecules diminish, and selenoenzyme synthesis is abolished, leading to a sustained loss of redox control. Thus, even a brief exposure to excess mercury may result in a self-perpetuating deficiency in selenoenzyme synthesis, termed the "selenium tailspin."
Technology Commercialization, Education, and Publication. To facilitate the transfer of technical information produced by CATMSM, several communication vehicles are used, including participation in conferences, symposia, workshops, educational programs, annual meetings, peer reviews, and the publication of a semi-annual newsletter.
The EERC, through CATMSM, EPA, and the U.S. Department of Energy, has organized and sponsored three conferences on Air Quality: Mercury, Trace Elements, and Particulate Matter, held in 1998, 2000, and 2002. The first conference was held in Tysons Corner, VA, and the second in McLean, VA. In 2002, the third conference was held in Arlington, VA. Air Quality IV: Mercury, Trace Elements, and Particulate Matter is scheduled to be held on September 22-24, 2003, in Arlington, VA. The Air Quality Conference is a forum for reviewing the current state-of-the-science and policy on the pollutants mercury, trace elements, and PM in the environment.
The CATMSM Web Site has been maintained throughout
the year and can be accessed at www.undeerc.org ![]() .
Copies of the CATMSM Newsletter are
available, as well as download access to the CATMSM
database. The CATMSM Web Site is continuing
to undergo major reconstruction and updates.
.
Copies of the CATMSM Newsletter are
available, as well as download access to the CATMSM
database. The CATMSM Web Site is continuing
to undergo major reconstruction and updates.
Journal Articles: 52 Displayed | Download in RIS Format
| Other center views: | All 347 publications | 110 publications in selected types | All 52 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Benson S. Air quality conference status of research on mercury. Filtration & Separation 1999;36(6):4. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Benson SA, Erickson TA, Jensen RR, Laumb JD. Transformations model for predicting size and composition of ash during coal combustions. American Chemical Society Fuel Preprints 2002;47(2):796-798. |
R827649 (2002) R827649 (Final) |
not available |
|
|
Benson SA. Air quality III: mercury, trace elements, and particulate matter. Fuel Processing Technology 2004;85(6-7):423-424. |
CR830929 (2004) R827649 (2003) R827649 (Final) |
not available |
|
|
Benson SA, Laumb JD, Crocker CR, Pavlish JH. SCR catalyst performance in flue gases derived from subbituminous and lignite coals. Fuel Processing Technology 2005;86(5):577-613. |
CR830929 (2005) |
|
|
|
Benson SA, Laumb JD, Jensen RR, Eylands KE. Characterization of particulate matter collected with a Burkhard sampler. American Chemical Society Fuel Preprints 2001;46(1):296-299. |
R827649 (Final) |
not available |
|
|
Biswas P, Senior C, Chang R, Vidic R, Laudal D, Brown T. Mercury measurement and its control: What we know, have learned, and need to further investigate. Journal of the Air & Waste Management Association 1999;49(12):1469-1473 |
R827649 (Final) |
not available |
|
|
Boschee P. EPA mercury regime risks apples-to-oranges results. Electric Light & Power 1999;77(2). |
R827649 (2000) R827649 (Final) |
not available |
|
|
Dronen LC, Moore AE, Kozliak EI, Seames WS. An assessment of acid wash and bioleaching pre-treating options to remove mercury from coal. Fuel 2004;83(2):181-186. |
CR830929 (2004) R827649 (2003) R827649 (Final) |
|
|
|
Galbreath KC, Zygarlicke CJ, Olson ES, Pavlish JH, Toman DL. Evaluating mercury transformation mechanisms in a laboratory - scale combustion system. Science of the Total Environment 2000;261(1-3):149-155. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Galbreath KC, Zygarlicke CJ. Mercury transformations in coal combustion flue gas. Fuel Processing Technology 2000;65:289-310. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Galbreath KC, Toman DL, Zygarlicke CJ, Huggins FE, Huffman GP, Wong JL. Nickel speciation of residual oil fly ash and ambient particulate matter using X-ray absorption spectroscopy. Journal of the Air & Waste Management Association 2000;50(11):1876-1886. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Galbreath KC, Toman DL, Zygarlicke CJ, Pavlish JH. Trace element partitioning and transformations during combustion of bituminous and subbituminous U.S. coals in a 7-kW combustion system. Energy & Fuels 2000;14(6):1265-1279. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Galbreath KC, Crocker CR, Nyberg CM, Huggins FE, Huffman GP, Larson KP. Nickel speciation measurements of urban particulate matter: method evaluation and relevance to risk assessment. Journal of Environmental Monitoring 2003;5(3):56N-61N. |
R827649 (2003) R827649 (Final) |
not available |
|
|
Galbreath KC, Crocker CR, Nyberg CM, Huggins FE, et al. Nickel speciation of urban particulate matter from Davie, Florida. American Chemical Society, Division of Fuel Chemical 2003;48(2):779-781. |
R827649 (2003) R827649 (Final) |
not available |
|
|
Galbreath KC, Zygarlicke CJ. Formation and chemical speciation of arsenic-, chromium-, and nickel-bearing coal combustion PM2.5. Fuel Processing Technology 2004;85(6-7):701-726. |
R827649 (2002) R827649 (2003) R827649 (Final) CR830929 (2004) |
|
|
|
Galbreath KC, Zygarlicke CJ, Tibbetts JE, Schulz RL, Dunham GE. Effects of NOx, α-Fe2O3, γ-Fe2O3, and HCl on mercury transformations in a 7-kW coal combustion system. Fuel Processing Technology 2005;86(4):429-448. |
R827649 (2002) R827649 (2003) R827649 (Final) CR830929 (2004) CR830929 (2005) |
|
|
|
Galbreath KC, Schulz RL, Toman DL, Nyberg CM, Huggins FE, Huffman GP, Zillioux EJ. Nickel and sulfur speciation of residual oil fly ashes from two electric utility steam-generating units. Journal of the Air & Waste Management Association 2005;55(3):309-318. |
CR830929 (2005) |
|
|
|
Hassett DJ, Eylands KE. Mercury capture on coal combustion fly ash. Fuel 1999;78(2):243-248. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Hassett DJ, Heebink LV, Pflughoeft-Hassett DF. Potential for mercury vapor release from coal combustion by-products. Fuel Processing Technology 2004;85(6-7):613-620. |
R827649 (2002) R827649 (2003) R827649 (Final) CR830929 (2004) |
|
|
|
Heebink LV, Hassett DJ. Release of mercury vapor from coal combustion ash. Journal of the Air & Waste Management Association 2002;52(8):927-930. |
R827649 (2002) R827649 (Final) |
not available |
|
|
Jensen RR, Karki S, Salehfar H. Artificial neural network-based estimation of mercury speciation in combustion flue gases. Fuel Processing Technology 2004;85(6-7):451-462. |
CR830929 (2004) R827649 (2003) R827649 (Final) |
|
|
|
Gupta H, Benson SA, Fan L-S, Laumb JD, Olson ES, Crocker CR, Sharma RK, Knutson RZ, Rokanuzzaman ASM, Tibbetts JE. Pilot-scale studies of NOx reduction by activated high-sodium lignite chars: a demonstration of the CARBONOX process. Industrial and Engineering Chemistry Research 2004;43(18):5820-5827. |
CR830929 (2005) |
|
|
|
Laudal DL, Brown TD, Nott BR. Effects of flue gas constituents on mercury speciation. Fuel Processing Technology 2000;65:157-165. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Laudal DL, Pavlish JH, Graves J, Stockdill D. Mercury mass balances: a case study of two North Dakota power plants. Journal of the Air & Waste Management Association 2000;50(10):1798-1804. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Laudal DL, Thompson JS, Pavlish JH, Brickett LA, Chu P. Use of continuous mercury monitors at coal-fired utilities. Fuel Processing Technology 2004;85(6-7):501-511. |
R827649 (2003) R827649 (Final) CR830929 (2004) |
|
|
|
Laudal DL, Thompson JS, Pavlish JH, Brickett L, Chu P, Srivastava RK, Lee CW, Kilgroe J. Mercury speciation at power plants using SCR and SNCR control technologies. Environmental Manager. 2003;(February):16-22. |
R827649 (2002) R827649 (2003) R827649 (Final) R827649C001 (Final) |
not available |
|
|
Laumb JD, Benson SA, Olson EA. X-Ray photoelectron spectroscopy analysis of mercury sorbent surface chemistry. Fuel Processing Technology 2004;85(6-7):577-585. |
R827649 (2002) R827649 (2003) R827649 (Final) CR830929 (2004) |
|
|
|
Mann MD. Mercury emissions. FGD and DeNOx Newsletter, May 1999, No. 253, pp. 5-6. |
R827649 (2000) |
not available |
|
|
Miller SJ, Dunham GE, Olson ES, Brown TD. Flue gas effects on a carbon-based mercury sorbent. Fuel Processing Technology 2000;65:343-363. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Olson ES, Miller SJ, Sharma RK, Dunham GE, Benson SA. Catalytic effects of carbon sorbents for mercury capture. Journal of Hazardous Materials 2000;74(1-2):61-79. |
R827649 (2001) R827649C001 (2001) R827649C001 (Final) |
not available |
|
|
Olson ES, Sharma RK, Pavlish JH. On the analysis of mercuric nitrate in flue gas by GC-MS. Analytical and Bioanalytical Chemistry 2002;374(6):1045-1049. |
R827649 (2002) R827649 (Final) |
not available |
|
|
Olson ES, Laumb JD, Benson SA, Dunham GE, Sharma RK, Mibeck BA, Miller SJ, Holmes MJ, Pavlish JH. Chemical mechanisms in mercury emission control technologies. Journal of Physique IV 2003;107(4):979-982. |
R827649 (2003) R827649 (Final) |
not available |
|
|
Olson ES, Laumb JD, Benson SA, Dunham GE, et al. The multiple site model for flue gas-mercury interactions on activated carbons: the basic site. American Chemical Society, Division of Fuel Chemical 2003;48(1):30-31. |
R827649 (2003) R827649 (Final) |
not available |
|
|
Olson ES, Crocker CR, Benson SA, Pavlish JH, Holmes MJ. Surface compositions of carbon sorbents exposed to simulated low-rank coal flue gases. Journal of the Air & Waste Management Association 2005;55(6):747-754. |
R827649 (2003) R827649 (Final) CR830929 (2004) CR830929 (2005) |
|
|
|
Pavlish JH. Status of particulate matter research and development. Filtration & Separation 1999;36(2):11. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Pavlish JH, Sondreal EA, Mann MD, Olson ES, Galbreath KC, Laudal DL, Benson SA. State review of mercury control options for coal-fired power plants. Fuel Processing Technology 2003; 82(2-3):89-165. |
R827649 (2002) |
not available |
|
|
Pavlish JH, Sondreal EA, Mann MD, Olson ES, Galbreath KC, Laudal DL, Benson SA. Status review of mercury control options for coal-fired power plants. Fuel Processing Technology 2003;82(2-3):89-165. |
R827649 (2003) R827649 (Final) |
not available |
|
|
Pavlish JH, Holmes MJ, Benson SA, Crocker CR, Galbreath KC. Application of sorbents for mercury control for utilities burning lignite coal. Fuel Processing Technology 2004;85(6-7):563-576. |
R827649 (2003) R827649 (Final) CR830929 (2004) |
|
|
|
Pflughoeft-Hassett DF, Hassett DJ, Heebink LV, Buckley TD. The current state of the science related to the re-release of mercury from coal combustion products. Ash at Work 2006;1:26-27. |
CR830929 (2006) |
not available |
|
|
Raymond LJ, Ralston NVC. Mercury: selenium interactions and health implications. Seychelles Medical and Dental Journal 2004;7(1):72-77. |
R827649 (Final) CR830929 (2004) |
|
|
|
Sondreal EA, Benson SA, Pavlish JH. Status of research on air quality: mercury, trace elements, and particulate matter. Fuel Processing Technology 2000;65:5-19. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Sondreal EA, Benson SA, Hurley JP, Mann MD, Pavlish JH, Swanson ML, Weber GF, Zygarlicke CJ. Review of advances in combustion technology and biomass cofiring. Fuel Processing Technology. 2001;71(1-3):7-38. |
R827649 (2001) R827649C001 (2001) R827649C001 (Final) |
not available |
|
|
Sondreal EA, Jones ML, Groenewold GH. Tides and trends in the world’s electric power industry. The Electricity Journal 2001;14(1):61-79. |
R827649 (Final) |
not available |
|
|
Sondreal EA, Benson SA, Pavlish JH, Ralston NV. An overview of air quality III: mercury, trace elements, and particulate matter. Fuel Processing Technology 2004;85(6-7):425-440. |
CR830929 (2004) R827649 (2003) R827649 (Final) |
|
|
|
Thompson JS, Pavlish JH. Cryogenic trapping of oxidized mercury species from combustion flue gas. Fuel Processing Technology 2000;65:167-175. |
R827649 (2000) R827649 (Final) |
not available |
|
|
Timpe RC, Mann MD, Pavlish JH. Organic sulfur and hap removal from coal using hydrothermal treatment. Fuel Processing Technology 2001;73(2):127-141. |
R827649 (2000) R827649 (2001) R827649C001 (2001) R827649C001 (Final) |
not available |
|
|
Xin M, Gustin MS, Ladwig K, Pflughoeft-Hassett DF. Air-substrate mercury exchange associated with landfill disposal of coal combustion products. Journal of the Air & Waste Management Association 2006;56(8):1167-1176. |
CR830929 (2006) |
|
|
|
Zhao Y, Mann MD, Pavlish JH, Mibeck BAF, Dunham GE, Olson ES. Application of gold catalyst for mercury oxidation by chlorine. Environmental Science & Technology 2006;40(5):1603-1608. |
CR830929 (2004) CR830929 (2005) CR830929 (2006) R827649 (Final) |
|
|
|
Zhao Y, Mann MD, Olson ES, Pavlish JH, Dunham GE. Effects of sulfur dioxide and nitric oxide on mercury oxidation and reduction under homogeneous conditions. Journal of the Air & Waste Management Association 2006;56(5):628-635. |
CR830929 (2004) CR830929 (2005) CR830929 (2006) |
|
|
|
Zhuang Y, Thompson JS, Zygarlicke CJ, Pavlish JH. Development of a mercury transformation model in coal combustion flue gas. Environmental Science & Technology 2004;38(21):5803-5808. |
CR830929 (2004) R827649 (Final) |
|
|
|
Zhuang Y, Zygarlicke CJ, Galbreath KC, Thompson JS, Holmes MJ, Pavlish JH. Kinetic transformation of mercury in coal combustion flue gas in a bench-scale entrained-flow reactor. Fuel Processing Technology 2004;85(6-7):463-472. |
R827649 (2003) R827649 (Final) CR830929 (2004) |
|
|
|
Zygarlicke CJ, Zhuang Y, Galbreath KC, Thompson JS, Holmes MJ, Tibbetts JE, Schulz RL, Dunham GE. Experimental investigation of mercury transformations in pilot-scale combustion systems and a bench-scale entrained flow reactor. Fuel Processing Technology. |
R827649 (2002) |
not available |
air, toxic, air quality, control, modeling, database, emissions, environment, hazardous, mercury, metals, pollutants, pollution, sampling, measurement, species, transformations. , Air, Sustainable Industry/Business, Scientific Discipline, Waste, RFA, Engineering, Chemistry, & Physics, Chemical Engineering, Analytical Chemistry, Incineration/Combustion, air toxics, Environmental Engineering, cleaner production/pollution prevention, Environmental Chemistry, Environmental Monitoring, combustion contaminants, emissions contol engineering, clean technology, combustion waste recovery, ambient air quality, cleaner production, ambient emissions, atmospheric models, combustion byproducts, combustion technology, combustion control, emission control strategies, hazardous air pollutants, aerosol particles, trace metal emissions, combustion, chemical kinetics of incineration, air pollutants, air quality models, emission control technologies, environmentally friendly technology, atmospheric chemistry, mercury, metals, ambient metal species, air pollution control, energy efficiency, metal vapor emissions, emission controls, green technology, pollution prevention, air emissions
Relevant Websites:
http://www.undeerc.org ![]()
http://www.undeerc.org/catm/catm_home.html ![]()
Progress and Final Reports:
2000 Progress Report
2001 Progress Report
Original Abstract
2003 Progress Report
Final Report
Subprojects under this Center:
(EPA does not fund or establish subprojects; EPA awards and manages the overall grant for this center).
R827649C001 Development And Demonstration Of Trace Metals Database
R827649C002 Nickel Speciation Of Residual Oil Ash
R827649C003 Atmospheric Deposition: Air Toxics At Lake Superior
R827649C004 Novel Approaches For Prevention And Control For Trace Metals
R827649C005 Wet Scrubber System
R827649C006 Technology Commercialization And Education
R827649C007 Development Of Speciation And Sampling Tools For Mercury In Flue Gas
R827649C008 Process Impacts On Trace Element Speciation
R827649C009 Mercury Transformations in Coal Combustion Flue Gas
R827649C010 Nickel, Chromium, and Arsenic Speciation of Ambient Particulate Matter in the Vicinity of an Oil-Fired Utility Boiler
R827649C011 Transition Metal Speciation of Fossil Fuel Combustion Flue Gases
R827649C012 Fundamental Study of the Impact of SCR on Mercury Speciation
R827649C013 Development of Mercury Sampling and Analytical Techniques
R827649C014 Longer-Term Testing of Continuous Mercury Monitors
R827649C015 Long-Term Mercury Monitoring at North Dakota Power Plants
R827649C016 Development of a Laser Absorption Continuous Mercury Monitor
R827649C017 Development of Mercury Control Technologies
R827649C018 Developing SCR Technology Options for Mercury Oxidation in Western Fuels
R827649C019 Modeling Mercury Speciation in Coal Combustion Systems
R827649C020 Stability of Mercury in Coal Combustion By-Products and Sorbents
R827649C021 Mercury in Alternative Fuels
R827649C022 Studies of Mercury Metabolism and Selenium Physiology
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20081106060834im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)