Final Report: Adsorption and Release of Contaminants onto Engineered Nanoparticles
EPA Grant Number: R831718Title: Adsorption and Release of Contaminants onto Engineered Nanoparticles
Investigators: Tomson, Mason B.
Institution: Rice University
EPA Project Officer: Lasat, Mitch
Project Period: August 1, 2004 through July 31, 2007
Project Amount: $333,797
RFA: Exploratory Research to Anticipate Future Environmental Issues: Impacts of Manufactured Nanomaterials on Human Health and the Environment (2003)
Research Category: Nanotechnology , Health Effects
Description:
Objective:This research focused on the evaluation of the sorption and release of contaminants onto the surfaces of engineered nanoparticles. Specifically, four hypotheses were tested in this research: 1. that carbon nanostructures have a high capacity for sorption/desorption hysteresis with polynuclear aromatic hydrocarbons and other common organic contaminants; 2. that the sorption capacity of inorganic nanomaterials for heavy metals is the same as the corresponding bulk crystals, when corrected for surface area; 3. that sorption of naturally occurring humic materials and surfactants to metal oxide and carbon nanomaterials will diminish the sorption capacity of heavy metals on oxides and increase the sorption of hydrocarbons on carbon nanomaterials; and 4. that the transport of nanoparticles in soils, sediments, and porous media is vastly greater than the corresponding colloids or bulk materials.
The overarching goal of the research was to create the information needed to ensure that avoidable and deleterious outcomes of nanomaterial production and use never materialize which may produce adverse health effects, halt manufacturing, and result in the need for costly remediation efforts. The overall objective of this project is to develop the information and models needed to understand and predict exposure routes of nanomaterials in natural systems.
Summary/Accomplishments (Outputs/Outcomes):The research group made substantial progress studying the adsorption of metals and organic compounds to both mineral and carbon engineered nanoparticles.
The transport or nano-C60 and associated contaminants in soil columns were studied. The nano-C60 colloidal particles prepared by direct sonication method, introduced in section 3.2.1, were adopted for the study of nano-C60 transport as well as the transport of 2, 2’, 5, 5’-tetrachloronated biphenyl (PCB) with nano-C60 in soil column transport experiments. The transport of solute in soil columns can be generally described by a one dimensional advective-dispersion equation.
A soil column packed with Lula soil (0.27% OC was pumped with nano-C60 particles in solution (0.01 M I and pH 8.33). Nano-C60 breakthrough curves at different Darcy velocities (from 1.22 m/day to 12.2 m/day) and one tracer (tritiated water) breakthrough curve at 1.22 m/day were plotted and breakthrough curves simulated with the analytical solution of a one dimensional convective dispersion equation by assuming local equilibrium and linear isotherms. The breakthrough curves of nano-C60 show that fullerene nanoparticles passed through soil column pore space and reach total breakthrough with an average retardation factor of 6.965±0.938 and a mean dispersivity (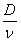 ) of 0.291±0.112 cm. It was found adsorption of nano-C60 to Lula soil follows a linear isotherm with partitioning coefficient (Kp) of 1.364 ml/g. By using this value and the porosity of 0.40 and the bulk density of soil of 1.57 g/cm3, a retardation factor R= 6.35 can be calculated for nano-C60 breakthrough, which is comparable with the results from column observation. The dispersivity is similar to that reported previously for Lula soil and is very reasonable for the porous media. In contrast with our earlier observations of sporadic breakthrough of nano-C60 in the same porous media, there is no evidence of “filter ripening”. This may be attributed to the differences in preparation method of aqueous nano-C60 suspension. The difference may be related to nC60 preparation differences, differences in solid packing, or a yet unknown effect.
) of 0.291±0.112 cm. It was found adsorption of nano-C60 to Lula soil follows a linear isotherm with partitioning coefficient (Kp) of 1.364 ml/g. By using this value and the porosity of 0.40 and the bulk density of soil of 1.57 g/cm3, a retardation factor R= 6.35 can be calculated for nano-C60 breakthrough, which is comparable with the results from column observation. The dispersivity is similar to that reported previously for Lula soil and is very reasonable for the porous media. In contrast with our earlier observations of sporadic breakthrough of nano-C60 in the same porous media, there is no evidence of “filter ripening”. This may be attributed to the differences in preparation method of aqueous nano-C60 suspension. The difference may be related to nC60 preparation differences, differences in solid packing, or a yet unknown effect.
Adsorption/Desorption of PCB to/from nano-C60 colloidal particles
The adsorption and desorption isotherm data was plotted for 2, 2’, 5, 5’-PCB to/from nano-C60 colloids. Sorption data can be fitted well with a linear isotherm with a partitioning coefficient (Kp) of 6.02*106 ml/g, suggesting that the sorption sites are homogenous. The observed Kp value in this study is similar to the Koc values for literature reported soils. Desorption experiments of PCB from nano-C60 was carried out in the same conditions. First, the solution was separated from nano-C60 solid phase by ultracentrifugation. The supernatant was removed and clean electrolyte solution was added to the centrifugation vials. The nano-C60 particles were re-dispersed in aqueous phase for one week and the vials were centrifuged again with a supernatant analyzed for PCB desorption. The amount of PCB desorbed is negligibly small, which may be due to slow desorption kinetics or duel equilibrium desorption. Desorption isotherms deviate considerably from the corresponding adsorption isotherms, suggesting the presence of hysteresis, i.e., desorption isotherm from each sample is not the reverse of the sorption isotherm. Chen et al proposed a dual equilibrium desorption model to explain the phenomenon of sorption/desorption hysteresis with a reversible and an irreversible compartment. In the reversible compartment, chemical could be readily and reversibly desorbed, while in the irreversible compartment, desorption of organic compounds could be hindered by soil organic matter. In terms of the mechanism of sorption hysteresis, pore deformation in carbonaceous materials has been discussed by many researchers. In many cases, the deformed pore structure did not recover to its original state even when the adsorbate was removed from the adsorbent, leading to the difference of desorption from adsorption. In this study, the hysteresis of PCB desorption from nano-C60 might be due to the deformation of micro pores in nano-C60 clusters. It is proposed that during the adsorption process, PCB molecules penetrate into the micro pores in nano-C60 clusters, resulting in the expansion of some pores due to the pressure exerted by adsorbate molecules. As a consequence, some available pore entrance will be blocked in the desorption process leaving some organic molecules entrapped.
PCB transport in soil column in the presence and absence of nano-C60 colloids
As for the highly hydrophobic organic contaminants, such as PCB, their mobility in soil is very limited, due to their strong sorption to soil, which is revealed by the calculated retardation factor of 2.3*107 in Lula soil. Based on experimentation, nano-C60 is capable of migrating through the same type of soil swiftly in less than 8 pore volumes, thus, it is expected to facilitate the transport of PCB.
The facilitated transport of hydrophobic organic compounds by colloidal particles can be mathematically described, where the hydrophobic organic compounds were assumed to undergo equilibrium partition between the mobile, dissolved organic carbon (DOC) phase and the stationary, soil organic carbon (OC) phase. If PCB transport in the presence of nano-C60 is governed by facilitated transport, the calculated PCB retardation factor should be around 2400, which is thousands of times faster than that for PCB alone.
An experimental comparison of the breakthrough of a PCB solution was made with a PCB solution containing nano-C60 through Lula soil at the same flow rate of 1.2 m/day. As for the PCB solution, no PCB was detected in effluent for 120 pore volumes of flow; while for the PCB-nanoC60 solution, the breakthrough curves of nano-C60 and PCB overlap, indicating simultaneous migration of PCB and nano-C60 in soil column. The experimentation results indicated that PCB molecules reached complete breakthrough after 7 PV, which is hundreds of times faster than that predicted by facilitated transport (2400).
The observation was made that the adsorbed PCB does not partition freely between aqueous phase and colloid surface in that PCB molecules have been entrapped in the micro pores of nC60 particles, which verified the presence of sorption hysteresis.
Environmental significance:
- As more and more carbon nanomaterials are manufactured, they will begin to appear in impacted soils and sediments. The potential impact of occurrence of hysteresis in nano-C60-hydrocarbon interactions on environmental fate and transport of carbonaceous nanomaterials and hydrocarbon pollutants could be enormous.
- The above results indicate that the carbonaceous nano materials once entered the environment may be capable of binding other environmental pollutants and causing wide spreading contamination of some pollutants which were previously considered non-mobile.
Heavy metal sorption to nano-magnetite
Before this study, little work had been conducted on the mixed oxide magnetite and its potential as a sorbent for arsenic, instead most literature focused on iron (III) oxides. This work has looked at several parameters that effect arsenic adsorption to magnetite nanoparticles such as, solid concentration, ions commonly found in water, and environmental conditions (pH, IS, DO). These results have contributed to the development of a model to predict arsenic adsorption in natural waters.
Adsorption results showed that arsenic adsorption increased with an increasing solid concentration and also with time. The results showed that arsenate and arsenite adsorption were similar to magnetite nanoparticles demonstrating the affinity of magnetite nanoparticles for both arsenate and arsenite. A first order rate equation was used to fit the adsorption results. And a correlation between the solid concentration and rate of adsorption was found to be linear, meaning if the solid concentration was doubled the rate of adsorption would be two times faster. From the calculated rate constants the mass transfer constants (km) were calculated to determine if adsorption was diffusion controlled or a surface reaction. The km values for arsenate and arsenite are similar, 1.21 ± 0.25∙10-7 m/s and 1.05 ± 0.20∙10-7 m/s, respectively. The mass transfer constant values are much smaller than typical values for diffusion controlled adsorption indicating that arsenic adsorption is surface reaction controlled. The adsorption results indicated that 0.5 g/L magnetite nanoparticles should be sufficient to remove arsenic and was further tested.
The presence of other ions in solution besides arsenic can affect arsenic adsorption; therefore, several ions commonly found in water were tested to determine their effect on arsenic adsorption to magnetite nanoparticles. The addition of ions to solution decreased arsenic adsorption, some having a larger effect than others. Phosphate at high concentrations, bicarbonate, and silica had the largest effect on arsenic adsorption. The results showed that phosphate is not a competing ion at concentrations typical for natural waters, which had not been previously shown by literature. Also, the results showed that there was no synergistic or additive effect when more than one ion was present in solution. These results were incorporated into a rate equation model that predicts arsenic adsorption to magnetite nanoparticles.
The effect of both ferrous and ferric iron was studied to determine if they had an effect on arsenic adsorption. Most natural waters have a fraction to a few milligrams per liter of iron present. The results showed that both ferrous and ferric iron increased arsenic adsorption but ferric iron had a greater effect. The ferric iron adsorption results were fitted and also incorporated into the model.
Several environmental conditions were tested because of their possible effect on arsenic speciation, arsenic adsorption, and the magnetite nanoparticle surface. At the narrow pH range tested, but representative of many tap and ground waters, a negligible effect on arsenic adsorption was observed. Results showed increasing ionic strength decreased arsenic adsorption. A possible explanation is when the ionic strength increases the electrostatic double layer around the magnetite nanoparticles decreases, potentially increasing particle aggregation and decreasing the available surface area for arsenic adsorption.
Based on the arsenic adsorption results a model was developed from a modified rate equation to predict arsenic adsorption to magnetite nanoparticles. The predicted results from the model were compared to measured results from adsorption experiments with Houston tap water and Brownsville, TX well water. The correlation between the predicted and measured results was very good (0.99). This model could be used for technology design to predict within one order of magnitude arsenic adsorption based on the water composition and solid concentration.
The results from this work show the viability of this method to treat arsenic contaminated water. Both Houston tap water and Brownsville, TX well water was treated using magnetite nanoparticles. Brownsville, TX well water was treated to below MCL within ten minutes with 0.5 g/L magnetite nanoparticles in the presence of high concentrations of several ions. The treatment method removed arsenic and other metals from solution showing its application as a platform technology for many contaminants. The well defined crystallinity of these particles allows them to be separated by either filtration or magnetic separation. However, magnetic separation offers a novel solution to removing the particles from solution.
Journal Articles on this Report: 8 Displayed | Download in RIS Format
| Other project views: | All 62 publications | 18 publications in selected types | All 17 journal articles |
| Type | Citation | ||
|---|---|---|---|
|
|
Chen W, Cong L, Hu H, Zhang P, Li J, Feng Z, Kan AT, Tomson MB. Release of adsorbed polycyclic aromatic hydrocarbons under cosolvent treatment: implications for availability and fate. Environmental Toxicology and Chemistry 2008;27(1):112-118. |
R831718 (Final) R828773 (Final) |
|
|
|
Lubick N. Tiny filters fix big water problems. Environmental Science & Technology 2007;41(13):4495-4496. |
R831718 (Final) |
|
|
|
Mayo JT, Yavuz C, Yean S, Cong L, Shipley H, Yu W, Falkner J, Kan A, Tomson M, Colvin VL. The effect of nanocrystalline magnetite size on arsenic removal. Science and Technology of Advanced Materials 2007;8(1-2):71-75. |
R831718 (Final) |
|
|
|
Tomson MB, Kan AT, Fu G. Control of inhibitor squeeze through mechanistic understanding of inhibitor chemistry. Society of Petroleum Engineers Journal 2006;11(3):283-293. |
R831718 (Final) R828773 (Final) |
|
|
|
Tomson MB, Kan AT, Fu G, Cong L. Measurement of total alkalinity and carboxylic acid and their relation to scaling and corrosion. Society of Petroleum Engineers Journal 2006;11(1):103-110. |
R831718 (Final) R828773 (Final) |
|
|
|
Tomson MB, Kan AT, Fu G, Al-Thubaiti M, Aramco S, Shen D, Shipley HJ. Scale formation and prevention in the presence of hydrate inhibitors. Society of Petroleum Engineers Journal 2006;11(2):248-258. |
R831718 (Final) R828773 (Final) |
|
|
|
Yang W, Duan L, Zhang N, Zhang C, Shipley HJ, Kan AT, Tomson MB, Chen W. Resistant desorption of hydrophobic organic contaminants in typical Chinese soils: implications for long-term fate and soil quality standards. Environmental Toxicology and Chemistry 2008;27(1):235-242. |
R831718 (Final) R828773 (Final) |
|
|
|
Yavuz CT, Mayo JT, Yu WW, Prakash A, Falkner JC, Yean S, Cong L, Shipley HJ, Kan A, Tomson M, Natelson D, Colvin VL. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006;314(5801):964-967. |
R831718 (Final) R828773 (Final) |
|
Health effects, bioavailability, nanotechnology, waste reduction, remediation, innovative technology, chemistry, environmental chemistry, nano-magnetite, arsenic removal, sorption,
,
POLLUTANTS/TOXICS, Water, INTERNATIONAL COOPERATION, TREATMENT/CONTROL, Sustainable Industry/Business, Scientific Discipline, RFA, Arsenic, Technology for Sustainable Environment, Sustainable Environment, Technology, Ecological Risk Assessment, Chemicals Management, Environmental Engineering, Water Pollutants, Environmental Chemistry, risk assessment, nanotechnology, environmentally applicable nanoparticles, fate and transport, environmental hazard assessment, clean technologies, bioacummulation, single walled carbon nanotubes, polynuclear aromatic hydrocarbons, green chemistry, engineering, nanoparticles, chemical behavior, nanomaterials, alternative materials
Progress and Final Reports:
2005 Progress Report
Original Abstract
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20081107170320im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)