ADE Surveillance: Evaluation Process
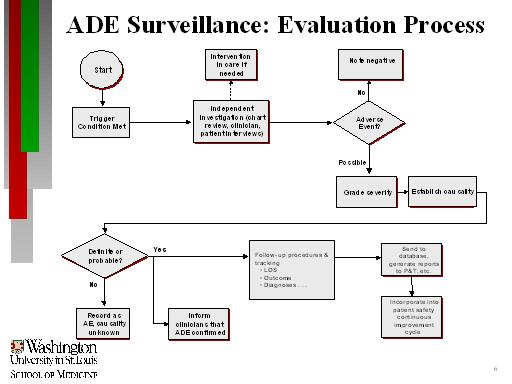
A flowchart maps out the evaluation process. To begin, a trigger condition is met. This leads to independent investigation (chart review, clinician patient interviews). There is an option of intervention in care if needed. The investigation leads to the question of whether an adverse event (AE) occurred. If no, note negative. If there possibly was an adverse event, the process must grade severity and establish causality. This leads to the question of whether causality was definite or probable. If no, record as AE causality unknown. If yes, there are two paths. The first path is to inform clinicians that ADE was confirmed. The second path involves follow-up procedures and tracking including LOS, outcome, diagnoses, etc. Then send to database, generate reports to P&T, etc., and incorporate into patient safety continuous improvement cycle.
 Previous Slide Contents Next Slide
Previous Slide Contents Next Slide 

