CBER Presentation
Accelerating the development & availability of vaccines for a pandemic or other emerging threats: present and future
Jesse L. Goodman, MD, MPH
Director, Center for Biologics Evaluation and Research (CBER), FDA
FDA Science Forum, Washington, DC, 4/20/06

Meeting the Pandemic Flu Vaccine Challenge: Ongoing FDA Actions
- Increasing manufacturing diversity & capacity
- Developing needed pathways to speed vaccine availability (e.g. guidances, accelerated approval)
- Facilitating vaccine manufacturing/evaluation/availability
- current and evolving technologies
- needed/improved assays, reagents, virus strains for development, manufacturing and release
- antigen sparing: adjuvants and delivery
- "holy grail" vaccines: cross-protective antigens
- considering priming and prevention strategies
- Global assistance, cooperation, harmonization
- Assuring safety and public confidence
Overview: Rapid Development and Deployment for Emerging Threats
- Big picture – how much risk vs. how much benefit?
- Where are we?
- Special tools to speed process
- So how quickly can we respond now?
- Two scenarios providing examples
- Promising evolving technologies and approaches
- A "roll out" concept for phased development and use?
- There are other important needs (e.g. immunization process itself and non-medical interventions)
Big picture – risk vs. benefit
- All medicine, public health and regulation is (or should be) risk/benefit based
- Challenge with developing and testing new vaccines for EIDs frequently is uncertainty of benefit (e.g. what is actual risk of disease) – evolves continuously and then changes dramatically when/if outbreak “arrives”
- FDA assesses risk/benefit for each product/use in context of the situation on the ground at that time
- Treatment: for otherwise untreatable, serious illness, reasonable to tolerate significant risk
- Prevention: for well individuals, balance shifts, especially if pre-exposure (or pre-outbreak)
- Lack of efficacy can be a safety issue
- Something is not always better than nothing
- Ineffective therapy can inhibit development of effective therapies
Risk-benefit cont.
- All products themselves also have uncertain risks
- Unexpected events occur, even following appropriate development, clinical studies and review (e.g. GBS with Swine flu, IS with RotaShield)
- Even uncommon AEs can have large impacts in setting of broad immunization of healthy population (1/100,000 = 3500 deaths) – of course far less than the impact of a potential pandemic
- Uncertainty about risk can be reduced by:
- Appropriate initial studies and continuing data acquisition and analysis during use
- Use of technologic approaches with historic experience
- Quality and experience in manufacturing and product testing
- Objective and effective risk communication critical
Progress- yes- but flies in ointment
- Major determinants of innovation,production speed and capacity are:
- Economics of industry
- Vaccine industry and capacity stabilized-improving
- First blockbuster vaccines, investments in CT and influenza are helping but maintenance will be critical
- Limitations: EID market inherently uncertain - government dependent if actions to be taken ahead of time, when needed
- Limits of technology and manufacturing
- Science has provided many new tools
- Rapid detection and cloning of new antigens (e.g. PCR for SARS, RG for flu), new adjuvants, production methods, delivery systems, platform technologies
- Limitations: knowledge gaps and inexperience - many approaches not predictable for given application. Lack of redundancy and resilience in manufacturing base.
How quickly can we respond now?
- Not as fast as we like or may need to!
- Components of response:
- Isolation of agent
- Preparation of seed strain/antigen
- Pilot manufacturing (bulk, purification, formulation)
- Proof of concept
- Clinical immunogenicity/efficacy and safety data as needed
- Scaled manufacturing, bulk, purification
- Fill and finish, product testing
- Delivery and administration
- Production, testing and quality does take time

Tools to Speed Product Evaluation and Availability
- Early and frequent consultation
- Fast track
- Priority review
- Accelerated approval - surrogate
- Approval under “Animal Rule”
- Availability for emergency use under IND or Emergency Use Authorization (EUA)
“Animal Rule”
- Products to reduce or prevent serious conditions caused by exposure to lethal or permanently disabling toxic chemical, biological, radiological, or nuclear substances
- Human efficacy studies not feasible or ethical
- Use of animal data scientifically appropriate
Animal Rule (cont.)
- Still need human clinical data
- PK/immunogenicity
- Safety
- Approval subject to post-marketing studies
- Potential limitations
- No valid or comparable animal model of disease
- How to predictably bridge animal data to humans
- Confidence an issue, even with valid models
Studies may also become helpful in some cases for development of vaccines and therapeutics for animals( and vice versa)
Emergency Use Authorization (EUA):
- Sec. of HHS can declare emergency after Sec. of Defense, Homeland Security, or HHS determines an emergency (or potential for one) exists, affecting national security
- Sec. of HHS (FDA) can authorize use of product:
- For serious or life-threatening condition
- No adequate, approved, available alternative
- Known & potential benefits outweigh known & potential risks
EUA: Conditions of Authorization
- Inform health care workers or recipients, if feasible
- Product authorized specifically for emergency use
- Significant known & potential risks and benefits
- Alternatives
- Option to accept or refuse
- Authority for additional conditions, e.g., who may distribute or administer, data collection & analysis
Groundwork is Needed for Broad Emergency Use Under IND or EUA
- Product may be used very widely in multiple populations
- Therefore, should have reasonable evidence of safety and support for efficacy or likely surrogate
- Primary time challenge in development is typically proof of principle and making product consistently - rather than clinical studies or review
- If product can be made, core data can be generated rapidly – example GSK Fluarix: 900 patients/~1 month/
- This should be done before emergency (or pre-pandemic/epidemic) wherever possible
- Managed, prioritized, funded processes needed to identify and develop candidates, assure data will be available to support use in an emergency
How Quickly: continued
- Time and data needs in each stage depend on disease and vaccine specific factors
- Experience with similar/related pathogens re: biology and protective response correlates
- Experience/capacity with needed technology, related vaccine(s)
- Clinical data needs: immunogenicity/effectiveness and safety
- Disease/host specific challenges, unknowns/concerns
- Two illustrative possible scenarios
- Fast: High experience, likely correlate, similar vaccines made, substantial capacity, no special concerns
- Moderate - uncertainties e.g. biology and/or correlate not understood and/or special concerns
- And then there are "black holes" e.g. retrovirus, prion disease
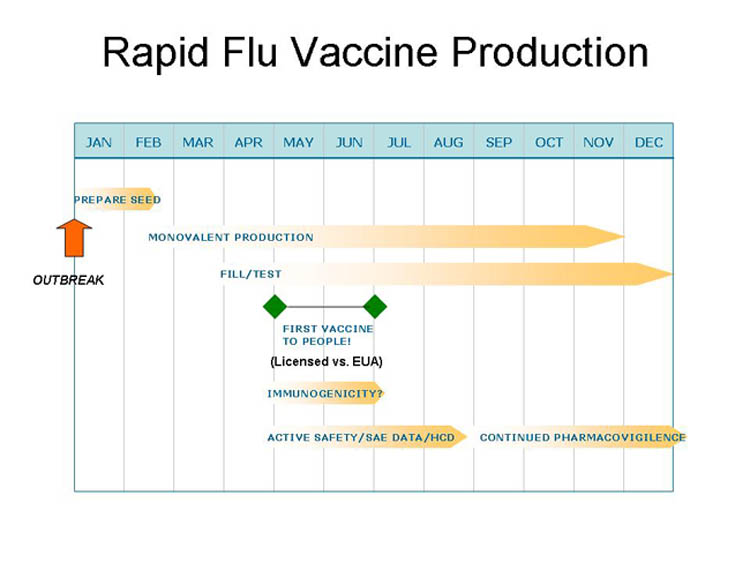
"Fast": New Influenza Strain
- Positives:
- High familiarity, annual experience
- Many licensed processes/facilities
- Ability to rapidly obtain antigen and develop seed strain
- Good safety record, limited need for clinical data for existing processes/vaccines
- Likely immune surrogate and bridge to effective licensed vaccines
- Negatives:
- Rapid antigenic changes and egg based technologies difficult to scale up
- Limited industrial capacity
- Manufacturing risks
- Testing for contamination important
Moderate - ?: SARS CoV
- Positives:
- Animals and people make protective neutralizing antibodies
- Small animal and primate models developed
- Negatives:
- No familiarity, experience, licensed products
- Possible safety concern- Ab dependent enhancement
- Killed vaccines not maximally effective
- Major proteins have complex glycosylation
- Need for immunogenicity and safety data – lack of correlate
- Animal disease models imperfect
Vaccine technologies to accelerate production or improve immunogenicity
- Reverse vaccinology (sequence based) - prior to culture
- Immunogen identification technology– xreactive epitopes
- Reassortants, reverse genetics*
- Cell culture* - scalability, potential use of contract facility
- Live atten* - rapid, broad antigenicity, Ab+CTL but safety
- Viral/bacterial vectored: "
- DNA (poor human responses) & "prime/boost": Ab + CTL
- Recombinant protein(s)*: mono-multi-antigen
- insect/animal cells for glycosylation
- plant, edible: dosing, environmental issues
- Synthetic or natural peptide*: "
- Virosome/pseudovirus/liposome – Ab + CTL
SARs: Virus Like Particles

Platform Technologies
- Use of standard platform as cassette/carrier for immunogen
- Examples: gene cassette, viral vector, virosome
- Potential benefits:
- with adequate experience, likely to gain predictability in safety, immunogenicity
- Speed of development/production
- Problem: not there yet- but worthwhile to systematically explore/invest
Adjuvants
- Enhance immune response- quantity +/- quality
- Highly variable in actions and effectiveness
- Increased potency often correlates with reactogenicity
- Mineral salts (e.g. alum) – most widely used Ab
- Emulsions/oils may be stronger adjuvants and stimulate more cross-reactive Abs and Th-1 CTL
- MF59 licensed flu vax in Europe
- Microbial "derivatives" (e.g. lipid A, CpG, toxins) – most stimulate innate immunity through different TLRs
- Microparticles and virus like particles – traffic antigen to APC's, can also serve as platform vectors/adjuvants
- Cytokines
Delivery Routes/Systems
- May enhance local humoral and cellular immunity, invoke APC and elicit CTL responses
- May allow more rapid practical delivery, delivery outside of health care settings, or self-immunization
- May conserve antigen
- Transcutaneous
- Mucosal
- Oral
Urgent Use? - Relevant Lessons of Swine Flu
- Communication re: benefit/risks critical
- Includes uncertainty of pandemic/epidemic - as vaccine benefit depends on it
- Likely better in non-crisis or routine situation - priming
- Ability and process to reevaluate changing situations
- Public's safety concerns and expectations are important and significant (and even more so today) and can affect, and even derail, vaccination plans
- Importance of safety monitoring in use
- Confidence in vaccines, governments and public health systems will be on the line
Relevant Lessons of CT Efforts
- Vaccine production complex, time consuming, not always predictable- vaccines are not widgets.
- Short-cuts seldom are.
- Less expensive seldom is.
- FDA and other global regulatory counterparts can play important and facilitating roles
- Help facilitate production, maximize the efficiency of investments
- Rapidly and objectively evaluate scientific findings re: safety, manufacturing and efficacy in face of urgency
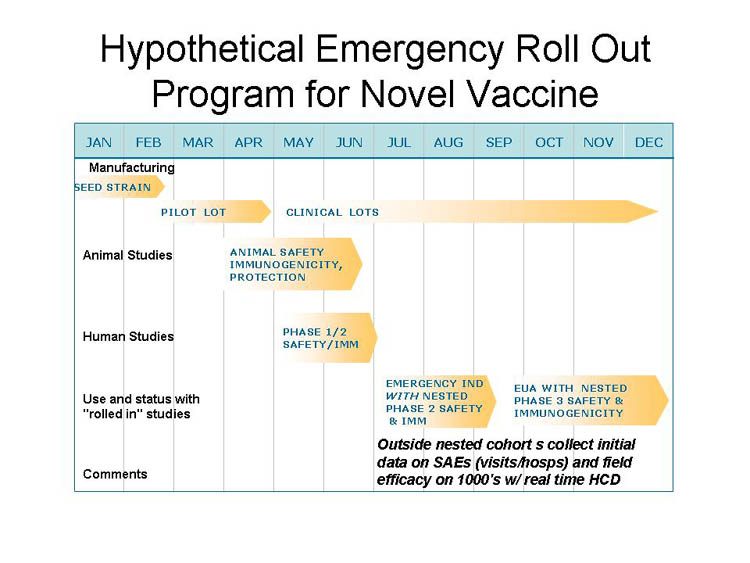
A New Conceptual Framework: "Roll Out"?
- In true or evolving emergencies, even accelerated vaccine development and evaluation approaches likely to fall short
- Can we integrate and speed the process through a "roll out" approach coordinating manufacturing and broadening clinical studies with initial use?
- In any case, effective deployment, roll out, and data acquisition of safety and efficacy studies will be needed for new products early during emergency availability
Conclusions
- Much accomplished and ongoing to improve vaccine technologies and nimble evaluation & regulatory pathways
- Many promising innovations are not yet "solutions"
- Even best case scenarios require months
- Thus, while success possible, we must also focus on:
- Enhanced surveillance and predictive sciences
- Ahead of time vaccine development against possible threats
- Better and predictive understanding of rapid platform vaccine technologies, adjuvants and manufacturing approaches
- Technologies to overcome antigenic variation, enhance stability
- Broad anti-infectives and nonspecific immune enhancement
- Development and evaluation of early non-medical interventions (e.g. personal protection/masks, social measures etc.)
- Much can be evaluated ahead of time - during annual flu seasons, for example
Thanks!
CBER: INNOVATIVE TECHNOLOGY ADVANCING PUBLIC HEALTH
Your input welcome - jgoodman@cber.fda.gov or 301-827-0372


