CBER Presentation
Risks versus benefits related to the possible implementation of a malaria blood-screening test
FDA Workshop on Testing for Malarial Infections in Blood Donors
July 12, 2006
Steve Anderson
Office of Biostatistics & Epidemiology
FDA-Center for Biologics Evaluation and Research
Probabilistic Modeling Risk/Benefit of New Donor Populations
- Current U.S. policy includes deferral of:
- Travelers - malaria endemic countries in last year
- Immigrants - from malaria endemic countries < 3 yrs
- Donors that had malaria - asymptomatic < 3yrs
- Goal: Use probabilistic model to evaluate potential risks / benefits and uncertainties of:
- Current policy
- Universal NAT Testing Scenario
- Universal Antibody Testing Scenario
Probabilistic Modeling
- Rather than single numbers or "point estimates"
- Employs statistical distributions for INPUT PARAMETERS - represents uncertainty of data
- Monte Carlo method chooses a value from each distribution as the "single number" for ONE iteration and generates OUTPUT as distributions
- Model is run thousands or millions of iterations and single "aggregate" OUTPUT distributions reflecting uncertainty and variability are generated
Uncertainty
- Arises from lack of or limited data for an input parameter(s)
- Assumptions used in model - add to uncertainty
- Lack of information or data for estimating -
- Self deferral for travelers to / immigrants from malaria areas,
- effectiveness malaria deferrals,
- Donation rates of travelers / immigrants,
- NAT test sensitivity,
- Antibody test sensitivity, etc.
- Uncertainty represented as confidence intervals about mean estimated outcomes
Malaria Risk in the United States
- 1,325 reported cases of Malaria identified in the U.S. in 2004 (CDC, MMWR 2006)
- All but 4 cases imported
- ~ 50% cases were Plasmodium falciparum
- Transfusion transmitted malaria (TTM) rate is low
- ~ 0.25 cases per million units collected
Possible Risks (Costs) and Benefits of Malaria testing of blood
- Risks (Costs)
- Additional malaria units, transfusion transmitted malaria (TTM), etc.
- Costs of testing entire supply (>14 million units / yr)
- Costs of re-testing units
- Loss of blood donors and blood units
- Costs of recruiting donors
- Benefits
- Number of additional donors gained
- Detection of additional malaria units from non-deferred donors
Overview of Model Components
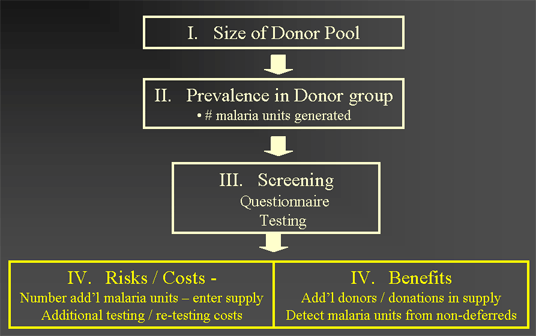
Estimation Size of Donor Pool
INPUT DATA:
- ~ 8 - 9 million Total Annual number blood donors
- ~ 27.4 million US travelers to malaria countries
- ~ 382,000 Immigrants from malaria countries
- ~ 60 % Population qualified to donate
- 5 % Donation rate general population
- 1.7 Annual donations per donor per yr
- ~ 14 million Total number blood donations per yr
OUTPUTS:
- > 880,000 Donors per year travel to malaria country
- > 730,000 Donors - self defer for malaria risk
- > 150,000 Donors - deferred by questionnaire
Estimation of malaria infection prevalence potential new donor groups
INPUT DATA :
- 95 - 99% Effectiveness of Questionnaire screen
(effectively lowers malaria prevalence in donors)
OUTPUTS:
- ~ 42 Potential mean malaria donors per year*
- ~ 71 Potential mean malaria donations per yr*
- ~ 3 Malaria units - not deferred per yr
*Most are removed by donor screening
Testing Scenarios: Universal Nucleic Acid Test (NAT)
- Test all donations using NAT
- Travelers (< 1yr) and Immigrants (< 3yr) to Malaria endemic countries
- Assumed there was a one month window period (WP) - donors with malaria not detected
- All other donors
- Assumed no window period
- Test Sensitivity assumed 99% - 100% sensitive
Testing Scenarios: Universal Antibody testing
- Travelers (≤ 3 months) to Malaria countries
- Assumed a 3 month WP - test may not detect malaria
- Travelers (> 3 months) to Malaria countries
- Test sensitivity - assumed to vary by species
- Immigrants (≤ 3yr) to Malaria countries
- Assumed no WP
- All other donors
- Assumed no WP
Travelers (> 3 months) to Malaria countries
- Adjust test sensitivities for (>3 mo) traveler population by occurrence of species in geographic regions traveled
(1) Assumed Test Sensitivity:
- P. falciparum 94% - 99.5%
- P. vivax 75% - 100%
- Others 50% - 75%
(2) Occurrence of species in travelers by region
| Pf | Pv | Other | |
| Africa | 82% | 10% | 7% |
| Asia | 11% | 83% | 6% |
| Americas | 36% | 57% | 6% |
| Others | 10% | 76% | 14% |
| All regions | 63% | 30% | 7% |
Results: potential risks and benefits of alternative screening methods
| Risks (5th, 95th perc) |
Benefits (5th, 95th perc) |
|||||
|---|---|---|---|---|---|---|
| CurrentPolicy | Blood units lost | Donors removed | Malaria units - not removed | Costs of screening | Malaria units removed | Potential donors gained |
| Self deferred | 1,276,000 | 729,000 | na | Assumed low
Costs for recruiting |
58 (48-79) |
na |
| Questionnaire deferred | 207,000 | 150,000 | 3 (1 - 5) |
9 (3 - 18) |
na | |
| Total: Self + Questionnaire | 1,483,000 | 879,000 | 3 (1 - 5) |
67 (48 - 90) |
na | |
Blood units collected per year in US = ~ 14 million
Results: potential risks and benefits of alternative screening methods
| Risks (5th, 95th perc) | Benefits (5th, 95th) | ||||||
|---|---|---|---|---|---|---|---|
| Blood units collected | Blood units lost | Donors removed | Malaria units - not removed | Costs of screening | Malaria units removed | Potential donors gained | |
| Current | ~ 14 million | 1,483,000 | 879,000 | 3 (1 - 5) |
Assumed low Costs for recruiting |
67 (48 - 90) |
na |
| NAT testing | 15,761,616 | 66 (46 - 87) (benefit) |
40 (30 - 51) (benefit) |
5 (2 - 9) |
Costs > 14 million tests Re-testing of units |
66 (46 - 87) |
~ 880,000 |
| Antibody testing | 15,760,264 | 1,418 (954 - 1912) (benefit) |
890 (600- 1200) (benefit) |
10 (4 - 16) |
Costs >14 million tests Re-testing of units |
61 (43 - 81) |
~ 880,000 |
Key Uncertainties
- Overall there is uncertainty for many of model inputs
- Would expect Malaria prevalence in donors with travel history (< yr) or immigrant - Malaria countries to be leading contributor to uncertainty
- Variability in malaria species by region over time
- Sensitivity of test that would by used
Conclusions from Malaria model
- Current policy - many donors (~ 150,000) deferred or ~ 880,000 donors if include self-deferrals
- Antibody testing - fewer donors deferred(~1,400)
- NAT testing - even fewer deferred (66)
- However, testing has significant costs associated with testing / re-testing >14 million units / yr
- But, testing scenario there may be a net gain of ~ 880,000 donors
- Need further exploration of costs of each option
- Testing
- Re-testing
- Recruitment of donors
- Validate assumptions (with data) on test sensitivities
- Peer review of Model
- Assumptions, data used, etc.
Acknowledgements
- Hong Yang - CBER/OBE
- Sanjai Kumar - CBER/OBRR
- OBRR staff


