Pancreatic Islet Transplantation to Treat Type I Diabetes
General Information - September 10, 2003
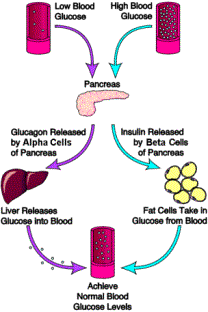 (Role of Insulin and Glucagon)9 |
Diabetes is a disorder in which the blood sugar levels are not regulated properly by the body. There are two major types of diabetes: type 1 and type 2. The following will focus on the use of islet transplantation to treat patients with type 1 diabetes and a history of difficult blood sugar control. While research has focused primarily on islet transplantation for type 1 diabetes, this treatment may eventually be considered as a viable option for all diabetic patients, both type 1 and type 2.1 |
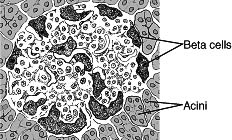
(Pancreatic Islet)10 |
Type 1 Diabetes:
Type 1 diabetes, also called juvenile diabetes or insulin-dependent diabetes, is a disorder of the body's immune system. In type 1 diabetes, the body's immune system attacks and destroys cells in the pancreas called beta cells. These cells are contained within small islands of endocrine cells called pancreatic islets. Beta cells produce insulin, a hormone that helps the body move the glucose contained in food into cells throughout the body, which use it for energy. When the beta cells are destroyed, insulin cannot be produced, and the glucose stays in the blood instead, where it can cause serious damage to the different organ systems of the body. People with type 1 diabetes face the constant threat of developing serious complications such as heart disease, blindness, kidney failure, and nerve damage to the lower legs. For this reason, people with type 1 diabetes have a real need for constant and continued treatment with insulin.
Generally, type 1 diabetes is diagnosed in children, teenagers, or young adults. Scientists believe that type 1 diabetes is directly related to autoimmune, genetic, and environmental factors.1,2
Treatment:
Current management of diabetes involves daily blood sugar testing, insulin injections, and careful meal planning. The only way to cure diabetes is to replace the destroyed beta cells or replace their function.
Whole-pancreas transplants have been successful in restoring insulin production in people with advanced diabetes, but because of significant risks the procedure has been limited to those who are also undergoing kidney transplantation.1,2
Islet Transplantation:
For decades, researchers have searched for ways to restore blood sugar control through human islet transplantation, a procedure in which only the islets that contain insulin-producing beta cells are transplanted. In 2000, the Edmonton Protocol brought attention to a novel approach for islet transplantation. This transplantation method used a larger quantity of islets and a combination of drugs that was less toxic to suppress the immune system.
Islet transplantation is a simpler and less invasive procedure than whole-organ transplantation. Healthy islets are isolated from a donor pancreas, purified, and then infused through a small tube into the portal vein of the liver. Patients take immunosuppressive drugs to keep their bodies from rejecting the new islets. When successful, islet transplantation can restore normal blood sugar without the need for insulin injections and can improve quality of life.
Islet transplants are still experimental. Therefore, they are available only to people who participate in a clinical study and meet specific criteria in the study protocol.3,4,5
Limitations of Islet Transplantation:
There is published information that several modifications of the Edmonton Protocol have been performed in over 15 centers involving over 160 patients worldwide. On average, patients who respond well to islet transplantation report being free of the need for insulin for about one year, with the longest case being 7 years. This is similar to the rate of insulin independence achieved following pancreas transplantation. Although the results from clinical studies appear promising, there are significant issues that remain before the technique can be considered for widespread application.3
- Limited Islet Supply: Based on the number of pancreas donors in the United States each year, only a limited number are suitable for transplant. The technique to isolate islets has not been perfected.
- Toxicity of Immunosuppression: Patients receive potent immunosuppressive medications for the rest of their lives after receiving a transplant. These medications are often associated with serious side effects. Therefore, patients also contend with a higher risk for infections resulting from a weakened immune system.
- Normal Blood Sugar Levels Not Achieved: Islet transplant patients appear to be able to have better control of their blood sugar levels compared to those who achieve it with insulin, diet, and exercise. However, only a small percentage of transplant patients achieve normal blood sugar levels.
- Long-Term Safety: Gaining access to the portal vein of the liver to transplant islet cells is a difficult procedure and involves some risks. The immediate risks include portal vein thrombosis and bleeding. The long-term consequences are not known, but reports of hepatic steatosis have been documented. This happens when fat globules collect within the cells of the liver and cause the tissue to deteriorate and malfunction.
- Duration of Islet Allograft Function: In addition to rejection by a patient's immune system, the transplanted islets are susceptible to exhaustion. Therefore, it is not known how long islets will function after transplantation, and whether the patient will need multiple transplants.
- Effect on Diabetic Complications: Controversy remains about whether a transplant can stop or reverse secondary complications related to diabetes. It is also not clear whether transplantation will ultimately extend a patient's long-term survival rate.
Other Research:
Patients who currently receive a transplant require lifelong treatment with potent immunosuppressive medications. These drugs are associated with many toxic side effects, and increase the risk of infection and cancer. Researchers are looking at ways to protect islets from being rejected after they have been transplanted without exposing patients to a lifetime of dangerous anti-rejection medications. They are looking at three possible strategies to achieve this: Block a specific reaction in the immune response pathway without compromising the entire immune system; mix immune cells from the source of islets with the immune cells of the recipient, in hopes that the recipient's immune system will recognize the donor cells as its own and not reject them; or give the recipient a low-dose therapy of immunosuppressive medications and let the surviving immune cells from the donor and the weakened immune cells of the recipient work together to fight infections.1,6
Researchers are also developing methods to slow or stop the autoimmune destruction of the insulin-producing beta cells of the pancreas. Current clinical trials with an investigational drug indicate that it can preserve beta cell function and improve blood sugar control during the first treatment year of type 1 diabetes.1,6
Other research is focused on using stem cells to generate new sources of islets for transplantation because there is a serious shortage of pancreata available for islet transplantation. The supply of donor pancreata from cadavers is much less than the estimated number of people with type 1 diabetes, approximately one million people in the United States. A few thousand pancreata are donated each year and, of these, only a limited portion is available for islet transplants. Therefore, new sources of islets that have been looked at include animal sources, surrogate cells engineered to mimic a beta cell's function, and embryonic or adult stem cells transformed into insulin-producing cells.1,2,4
Role of FDA:
Over the past 3 years, the Food and Drug Administration (FDA) has seen a significant increase in investigational new drug (IND) applications for the use of donor pancreata to treat type 1 diabetes. Recent finding have triggered a renewed interest in the potential long-term benefit for these patients through islet transplantation. The FDA invited outside experts to discuss manufacturing, pre-clinical, and clinical issues related to this topic at an advisory committee meeting in March 2000. Another meeting to continue discussion of these issues is scheduled for early October 2003.7
The clinical use of islets to treat type 1 diabetes meets the criteria for regulation as both a biologic product and a drug product. Therefore, clinical studies are needed for this experimental therapy in order to gather safety and effectiveness data in accordance with IND regulations set by FDA, and to ensure the safety and rights of patients in all phases of the investigation.7,8
Related Information:
Food and Drug Administration -
FDA Diabetes Information - http://www.fda.gov/diabetes/
Center for Devices and Radiological Health (CDRH) - Search for monitoring devices, advisory panel recommendations http://www.fda.gov/cdrh/
Biological Response Modifiers Advisory Committee Meeting on Pancreatic Islet Transplantation - March 20-21, 2000
Centers for Disease Control and Prevention (CDC) - http://www.cdc.gov/health/diabetes.htm
National Institutes of Health -
Islet Cell Resource Centers (ICRs) - http://www.ncrr.nih.gov/clinical/cr_icr.asp
National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) - http://www.niddk.nih.gov/
NIDDK Diabetes - http://diabetes.niddk.nih.gov/
Health Resources and Services Administration (HRSA) - http://www.hrsa.gov/ConsumerEd/
Immune Tolerance Network (ITN) - co-sponsored by the NIAID, NIDDK, and JDRF - http://www.immunetolerance.org/
Juvenile Diabetes Research Foundation International (JDRF) - http://www.jdrf.org
References:
- What is diabetes? Juvenile Diabetes Research Foundation International Web site: www.jdrf.org
- Diabetes information. Food and Drug Administration Diabetes Information Web site: www.fda.gov/diabetes
- Hirshberg B, Rother KI, and Harlan DM. Islet transplantation: where do we stand now? Diabetes Metab Res Rev. 2003;19:175-178.
- Pancreatic islet transplantation. National Institute of Diabetes and Digestive and Kidney Diseases Web site: www.niddk.nih.gov
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230-238.
- Islet transplantation. National Institute of Allergy and Infectious Diseases Web site: www.niaid.nih.gov
- Weber DJ, McFarland RD, and Irony I. Selected food and drug administration review issues for regulation of allogeneic islets of langerhans as somatic cell therapy. Transplantation. 2002;74(12):1816-1820.
- Zoon, KC (2000, September). Dear colleague letter to transplant centers-allogeneic pancreatic islets for transplantation. Center for Biologics Evaluation and Research Web site:http://www.fda.gov/cber/ltr/allpan090800.htm
- Role of insulin and glucagons. Endocrine Disorders and Endocrine Surgery Web site: www.endocrineweb.com
- Pancreatic islets. Merck Source Web site: www.mercksource.com


