Cellular Therapy
Potential Treatment for Heart Disease
Introduction
As novel cellular therapies move from laboratory findings to clinical practice, medical researchers and regulators face new issues and uncertainties involving long-term safety and efficacy.
Currently, there are no effective drug therapies for many acquired and congenital diseases. Recent discoveries in cellular therapy research present new opportunities for cellular products to be used in disease areas with critical, unmet medical needs. The Food and Drug Administration (FDA) regulates cellular therapies to ensure that they are safe and effective, and that persons enrolled in clinical trials using cellular products are protected from undue risk.
Cellular products hold great potential for use in treating damaged or diseased tissues of the body. Cellular products come from a variety of sources, such as stem cells from bone marrow and peripheral blood, and myoblasts from skeletal muscle cells.1, 2, 3
There is still much to learn about how cellular products work, how to administer them safely, and whether, over time, the cells will continue to work properly in the body without harmful side effects. FDA is committed to supporting cellular therapy research and development, and future licensure of cellular products, and wants the public to understand both the promise and the challenges presented by this exciting new therapy.
Stem Cell Research
Stem cells, regardless of origin, have the remarkable potential to develop into many different cell types in the body. When a stem cell divides, each new cell has the potential to either remain a stem cell or become another type of cell with a more specialized function, such as the beating cells of the heart.1, 2
There are two characteristics that distinguish stem cells from other types of cells. 1) Stem cells can self-renew, and 2) they can differentiate into other types of cells. Stem cells are unspecialized cells that renew themselves for long periods through cell division, and given certain conditions, can be induced to become cells with special functions.2
Stem cells have potential in many areas of medical research. One possible application is to make new cells and tissues for medical therapies. For example, donated organs and tissues are often used to replace those that are diseased or destroyed. Unfortunately, the number of people suffering from disorders that might benefit from stem cell therapy is much greater than the number of organs and tissues available for transplantation. Stem cells offer the possibility of renewable sources of replacement cells and new tissues to treat many kinds of diseases, conditions, and disabilities.1, 4
Cellular Therapy to Treat Heart Disease
Despite many recent advances in medical therapy and interventional techniques, ischemic heart disease and congestive heart failure (CHF) remain the major causes of morbidity and mortality in the United States.4, 5 Cellular therapy for treating these and other heart conditions is a growing field of clinical research. Potential cell treatments for patients with congestive heart failure (CHF) and ischemic heart disease are of great interest to medical researchers and treating physicians.
Research to date has involved cells from autologous (donors who are also the recipients of the cellular therapy) skeletal muscle*, hematopoietic stem cells from autologous peripheral blood, and unspecialized mesenchymal or hematopoietic stem cells from bone marrow. They have been administered through catheters into the coronary arteries, transendocardially through injection catheters into the left ventricular myocardium, or transepicardially through a needle during coronary artery bypass graft.3
* Myoblasts are muscle precursor cells that come from differentiated skeletal muscle cells. Because they are easily obtained and capable of regeneration, researchers are attempting to use myoblasts as a source of cells to repair the heart.
Issues
A diversity of opinion surrounds the feasibility of beginning clinical studies using human stem cells. Some scientists and medical researchers believe it is reasonable to expect that, within the next five years, human stem cells will be used to replace dead or dying cells within organs, such as the failing heart. Others argue that more information is needed about the basic biology of human stem cells before it is possible to assess their therapeutic potential.4
Before clinical studies involving cellular therapy can be done, it is essential to demonstrate that cellular products have the relevant biological activity - that the therapy does what it is supposed to do without being toxic.4, 5
Three important issues confronting the development of cellular products for the treatment of heart disease include manufacturing, catheter-cellular product interactions, and the nature and quantity of pre-clinical data needed to begin early phase clinical studies.3
Differences among individual cell donors can produce inconsistencies, even among products using the same manufacturing process. Different formulation and storage conditions may also have an effect on the cellular product.3
Many questions remain about the safety and mechanisms of action of stem cells for the treatment of heart diseases. Pre-clinical studies provide toxicity data, and may also provide useful data regarding the cellular product's mechanism of action.3
Certain in vitro studies have shown that non-heart cells may be manipulated to take on functional characteristics of heart cells. These transplanted cells may acquire the ability to revascularize, regenerate muscle and conduct electrical impulses in the heart.3 (See figure 1)
Heart catheterization methods and devices are being investigated as a way to deliver cellular products. Current research in this area is focused on development of heart catheters and methods that can provide targeted delivery of high concentrations of cells to specific regions of the heart muscle.3
Issues related to the safety and effectiveness of the medical devices used to deliver stem cells to a patient are still under investigation. For example, more understanding is needed regarding the safety and effectiveness associated with the delivery of cells by coronary artery balloon catheter devices.3 (See figure 2)
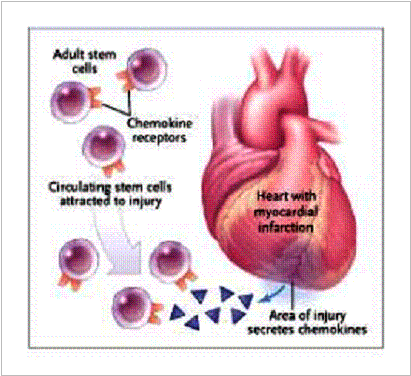
Figure 1. Homing Hypothesis
Rosenthal N. Prometheus' Vulture and the Stem Cell Promise. NEJM July 2003;349:267-274.
Adapted with permission by the Massachusetts Medical Society
Stem cells are hypothetically able to migrate into areas of muscle degeneration or injury, participate in the regeneration or repair process, and give rise to fully differentiated heart muscle fibers. These stem cells appear to be recruited by long-range, possibly inflammatory, signals originating from the degenerating or injured tissue. They appear to access the heart muscle from the blood circulation.10
Figure 2. Intracoronary Administration
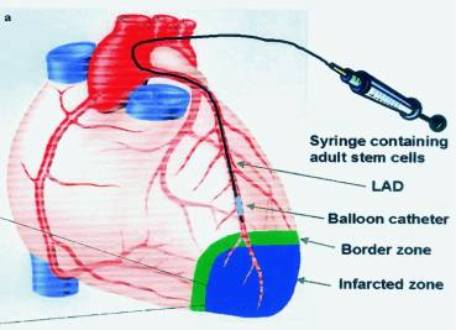
Strauer BE, et al. Repair of Infarcted Myocardium by Autologous Intracoronoary Mononuclear
Bone Marrow Cell Transplantation in Humans.
Circulation 2002;106:1913-1918.
Adapted with permission by the American Heart Association.
Role of FDA
The Food and Drug Administration (FDA) has a long history of effectively safeguarding the public health and strong experience in regulating drugs and biological products. FDA's regulatory framework is based, in part, on manufacturing procedures, the use of investigational devices in some studies, the different use of cellular products, and safety concerns associated with administration of these products.8, 9
FDA has developed a framework that provides a tiered approach to both cell and tissue regulation. This regulation focuses on three general areas to: 1) prevent the use of contaminated tissues or cells with the potential for transmitting infectious diseases such as AIDS and hepatitis; 2) prevent improper handling or processing that might contaminate or damage tissues or cells; and 3) ensure that clinical safety and effectiveness is demonstrated for tissues or cells that are highly processed, are used for other than their normal function, are combined with non-tissue components, or are used for metabolic purposes.5
FDA's main priority is to ensure safe human cellular therapy studies. FDA does not want to stand in the way of progress that may lead to effective treatments, but the Agency recognizes that new cellular therapies may have unknown risks, even as they provide benefits to patients.7
The regulatory pathway for cellular products is an evolving process and certain issues related to the licensure of cellular products are not resolved yet. FDA's Biological Response Modifiers Advisory Committee (BRMAC) will meet March 18-19, 2004 to discuss the scientific basis for clinical development of cellular products to be used in the treatment of heart diseases. FDA will hold future BRMAC meetings to discuss the regulatory issues associated with late phase clinical development of these cellular products.
References
Stem Cell Basics. NIH web site
Stem Cells: Scientific Progress and Future Research Directions. NIH web site
Briefing document: Cellular Products for the Treatment of Cardiac Disease. Biological Response Modifiers Committee Meeting #37. March 18-19, 2004.
Fink, DW. Assessing human stem cell safety. 2001 NIH Stem Cell Report.
-
Proposed Approach to Regulation of Cellular and Tissue-based Products (PDF - 434 KB). February 1997.
-
Scientists find new way to grow human embryonic stem cells. March 19, 2003. Science Daily web site.
-
Human Gene Therapy and The Role of the Food and Drug Administration. FDA web site.
-
Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy (PDF - 78 KB) . March 1998.
- Jensen GS and Drapeau C. The use of in situ bone marrow stem cells for the treatment of various degenerative diseases. Med Hypotheses 2002;59(4):422-428.


