|
|
 |
| Frequently Asked Questions
|
The following
questions were received over time from industrial hygiene professionals in laboratories
and the field. These include persons employed in foreign, federal, state and local
governments; private laboratories; manufacturers; industry; construction; and insurance.
If you have a question that does not appear in the list below, try using your browser's
"Find" feature (usually a control F command) to locate key words on this page.
Evaluating Exposure Levels:
How does the OSHA
Permissible Exposure Limit (PEL) of 10/(%Quartz + 2) compare to the American Conference of
Government Industrial Hygienists' (ACGIH) old Threshold Limit Value (TLV) of 0.1 mg/m3?
Most OSHA PELs are set values for a single air contaminant such as cadmium or a
related family of contaminants such as the polyaromatic hydrocarbons (PAHs). The PEL for
respirable dust containing quartz differs considerably in that it is a function that
varies between a value of 0.1 mg/m3(when the material is pure quartz) up to a
value approaching the Particulates Not Otherwise Regulated (PNOR) OSHA PEL of 5 mg/m3.
The PEL does not apply below 1% quartz, so the highest it can get is 3.3%. (When the
concentration drops below 1%, the PNOR PEL of 5 mg/m3 applies.) It can be shown
mathematically that when this PEL function for respirable dust containing quartz is
divided into the TWA exposure, the resulting standardized concentration (or exposure
severity) is the sum of the standardized concentrations for the separate quartz and PNOR
exposures. This derivation requires only simple algebra, but is available in the
literature [Frank J. Hearl, "Mixture Formula Justified," Letters to the Editor,
AIHA Journal 57, June 1996, p 575 and also in Frank J. Hearl, "Guidelines
and Limits for Occupational Exposure to Crystalline Silica," in Silica and
Silica-Induced Lung Diseases, V. Castranova, V. Vallyathan, and W. E. Wallace Eds., CRC
Press Inc. pp.15-22.] [Note that the derivations count the quartz twice because it is not
subtracted from the dust exposure in determining the PNOR exposure. If the quartz were
subtracted, the quartz standard of 0.098 mg/m3 would result, which is only very
slightly more restrictive than the 0.1 mg/m3 proposed in the (now vacated)
Final Rule.] Through the same approach, it is easy to derive the PEL for the mixture of
respirable dust containing quartz and cristobalite [PEL = 10/(%Quartz + 2(%Cristobalite) +
2)]. The use of these PELs achieves the same result as using the mixture calculation
specified in 29 CFR 1910.1000 for exposures to substances having an additive effect on the
body or target organ system.
How do I perform a crystalline silica mixture calculation when the sample contains
both quartz and cristobalite?
The Silica Advisor provides a discussion
and example of the calculations. An Advisor
Genius calculator is also available which will perform the calculations.
I am in the construction industry and can't locate any lab that analyzes impinger
samples collected for quartz in terms of millions of particles per cubic foot (mppcf)
using microscopy. How should I sample and analyze for quartz?
That methodology is obsolete. The impinger samples need to be counted within 24
hours. Errors associated with the analyses are unknown and the method does not provide
information about the particulate composition. Separate samples must be taken to determine
the % crystalline silica in the samples. Therefore, the microscopy method is
impractical. Instead, resample using the cyclone and collect on tared low-ash PVC filters.
Analyze by XRD, IR, or by a Chemical Method as appropriate for your sample matrix and
apply the equivalent OSHA PEL for respirable dust containing quartz, 10/(%Quartz + 2) in
terms of mg/m3. For details, see Appendix F of Memorandum of OSHA Secretary
Joseph A. Dear, May 2, 1996, Special Emphasis Program (SEP) for Silicosis.
How do I interpret a company's crystalline silica sampling data when it doesn't list
the percentage quartz and all I have are the TWA exposure mg/m3 in terms of
quartz and the corresponding TWA exposure mg/m3 for the mixture of respirable
dust containing quartz? For example, say I'm told that the quartz exposure was 0.066 mg/m3
and the mixture exposure was 3.0 mg/m3.
There are two mathematically equivalent ways you can determine the standardized
exposure of the mixture:
- From the response to FAQ #1 above, one could obtain the sum of the standardized
exposure to quartz at an exposure limit of 0.100 mg/m3 and the standardized
exposure to the mixture at a PEL of 5.0 mg/m3. In the example exposures given
above, the summed standardized exposure is 0.066/0.100 + 3.0/5.0 = 0.66 + 0.60 = 1.26.
This method may be how the company tracks exposures. The practical advantage is that the
component standardized exposures are determined separately, which may help in evaluating
the engineering actions needed to bring the exposure to the mixture under control. The
company may be using the 0.050 mg/m3 respirable quartz REL or TLV as a more
restrictive exposure limit; it is still important that it consider the additive effect of
the exposures to both quartz and PNOR.
- For compliance purposes calculate the percentage quartz by taking 100 times the
ratio of the TWA quartz exposure divided by the TWA mixture exposure. (The equal volumes
of air in the concentration units cancel out leaving a weight ratio.) In the example
exposures given above, the percentage quartz is 100 (0.066/3.0) = 2.2%. From this the PEL
is 10/(2+2.2) = 2.38 and the standardized exposure would be 3.0/2.38 = 1.26, which is the
same result as in (a) above.
Note: Calculating the standardized exposure both ways can provide a check on your math,
but results from methods (a) and (b) may differ slightly due to round off of intermediate
results.
How do I use the percentage quartz results from the analysis of the bulk samples to
determine whether the OSHA PEL for respirable dust containing quartz has been exceeded on
the air samples?
Please Don't. Analytical results on the quartz content of the air samples are
necessary to evaluate whether the OSHA PEL is exceeded. Results for bulk materials do not
necessarily represent the composition of the respirable dust suspended in the air at the
time of sampling. Bulk materials are analyzed primarily to establish the potential for
exposure or to assist the laboratory when interferences are encountered or may be present
in the air samples. See Memorandum of OSHA Secretary Joseph A. Dear, May 2, 1996, Special
Emphasis Program (SEP) for Silicosis, for recommendations on collection of bulk samples.
We need OSHA's sampling and analysis error (SAE) for the analysis of crystalline
silica in order to apply it to the results we get from our private contract lab. Where can
we obtain OSHA's SAE?
This request indicates some confusion with regard to industrial hygiene analyses
and the meaning of SAE. Generally speaking, neither NIOSH nor OSHA mandate either specific
analytical procedures or analytical equipment. Good science should prevail, and when
permitted, other methods may be used. Each IH laboratory should determine its own SAE
values based on the analysis of quality control samples, spiked samples or other tests of
recovery. The private contract lab should be contacted for its SAE estimates for the time
period of the analyses.
The SAE is not a universal constant but is a time-varying function of instrument and
analyst performance for particular analytes. The OSHA PEL for dust containing quartz is
given by PEL = 10 / (%Quartz + 2) , where the %Quartz in the respirable dust is calculated
from the analytically determined mass of quartz in the gravimetrically determined mass of
the dust. The standardized concentration is a measure of exposure severity to which the
SAE is applied to confidently determine whether an overexposure has actually occurred. The
standardized concentration is: Standardized Concentration = Exposure / PEL, where the
Exposure term refers to the gravimetrically measured time-weighted average concentration
(mg/m3) of respirable dust containing quartz. So the application of the SAE for
respirable dust containing quartz involves two analyses: Gravimetric and quartz analyses.
The OSHA SAE for quartz analysis is provided to the OSHA compliance officer in the same
report as the analytical results. Assuming a 5% error in the flow rate, the SAE for quartz
analysis is typically around 20% for 95% confidence.
So strictly-speaking, the SAE for such analyses contains weighted components for the
errors associated with the flow rate, gravimetric, and quartz portions of the analyses.
The function for the overall SAE (at 95% confidence) that can be derived for the combined
errors of the two analyses varies between about 10% for respirable dust with little quartz
to 20% for respirable dust that is nearly pure quartz. Using the SAE for quartz given with
the analytical report, therefore, provides a better than 95% confidence level. The PEL
calculation for respirable dust containing both quartz and cristobalite is given by:
PEL = 10 / [2 + %Quartz + 2(%Cristobalite)],
so a similar situation arises.
Regarding Air Sampling
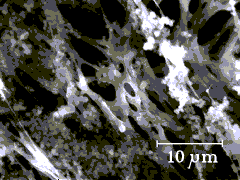
How does a PVC filter with a 5-micron pore size capture submicron size dust?
The low-ash 5-micron PVC filter is the preferred sampling medium for respirable
dust containing crystalline silica and is used in the American Industrial Hygiene
Association (AIHA) Performance Analytical Testing (PAT) Program. The 5-micron pore size
filters reduce problems associated with sample loading and back pressure. This condition
is important to maintaining a constant sampling rate in dusty work environments.
There are several reasons generally attributed as to why these filters work well in this application.
The pore size in this type of membrane filter is just one description of the filter
medium. The 5-micron size for this filter is not the physical dimension of holes at the
surface of the filter; it is based on the pressure drop across the filter. The electron
microscope shows the surfaces to be a maze of PVC strands presenting passageways much
smaller than 5 microns. The "smooth" surface appears as a spongy mat on which
impaction can occur. Pore paths are not linear so particles do impact surfaces.
| This
is an image of the smooth shiny side of clean 5-micron filter (Omega P503700). |
This
is the rough, dull side of the same clean filter. |
 |
 |
| Click
the image to enlarge. |
Click
the image to enlarge. |
Such filters do not behave merely like sieves and have good collection efficiencies for air
suspended dusts well below the pore size. The Omega Specialty Instruments 5-micron PVC
membrane filter P503700 is used in the MSA 800019 cassette in the OSHA automated weighing
program. In capture efficiency tests, the P503700, for example, retained 99.9% of
respirable coal dust. Particles are captured primarily by impaction and diffusion (Morris
Katz, Ed., Methods of Air Sampling and Analysis, 2nd Ed., American Public Health
Association, Washington, DC 1977, p 199 and University of Minnesota Short Course on
Aerosol and Particle Measurement, Aug. 22-24, 1994). Dust particles tend to agglomerate
(clump or stick together) due to Van der Waals, polar and ionic surface features, and
hydrogen-bonding forces. [Crystalline silica surfaces exposed to the air rapidly become
covered with silanol (Si-OH) groups that can ion exchange and hydrogen bond.] Through
agglomeration, smaller particles join together and with larger particles to be more
readily captured on the filter than isolated particles. Filter loading also increases
filter efficiency; as the filter begins to pick up dust, the effective pore size
decreases. Dusts are rarely spherical. For example, quartz dust produced by abrasive and
grinding processes generally appears under the microscope like shards of broken glass. So
in some orientations they often present a larger cross section for capture than their
equivalent spherical diameter may indicate. Most aerosols in the workplace carry a static
electrical charge. When they contact the filter they induce an opposite charge to form at
the surface enhancing the surface attraction. Also, uncharged particles can develop
charge; when different materials (the aerosol and the membrane) come in contact they
become oppositely charged and attracted to one another.
[Note: Currently available PVC
filters of the smaller pore size (0.5 micron) do not dissolve properly in tetrahydrofuran
(THF) and therefore cannot be analyzed by OSHA method ID-142.]
Can I sample crystalline silica using low-ash 0.4-micron pore size AA (MCEF) filters
instead of low-ash PVC filters?
Generally speaking, no.
The weights of MCEF filters are affected greatly by slight variations in humidity and
are not suitable for the gravimetric portion of the analysis of respirable dusts
containing crystalline silica.
Additionally, MCEF filters do not dissolve properly in THF and therefore the
crystalline silica collected on MCEF cannot be readily analyzed by OSHA method ID-142.
Nylon has a problem with developing a surface static charge during use. I've been
told that I can use a metal cyclone operated at a specific flow rate to give the desired
50% cutpoint at 3.5 microns. Why do we still use nylon cyclones rather than the newer
metal cyclones or conductive plastic cyclones?
Manufacturers often refer to the 50% cutpoint of 3.5 microns as being the critical
distinction, but the other cutpoints specified in 29 CFR 1910.1000 Table Z-3 are equally
important. These sampling efficiencies are at various aerodynamic particle sizes that
compare well with both the then-current model for respirable dust deposition in the human
respiratory tract and with the sampling efficiency of the AEC instrument consisting of a
1-cm Dorr-Oliver cyclone made with a nylon body. (The nylon body is not to be confused
with the sampler holder which may have a metal protective tube surrounding the cyclone
body.) Currently the only cyclone we are aware of that has the characteristic sampling
efficiencies listed in 29 CFR 1910.1000 Table Z-3 is the Dorr-Oliver nylon cyclone. A
device that does not develop a static charge would probably give more reproducible
sampling and represent an improvement, but until an equivalent conductive device becomes
available, we use the Dorr-Oliver nylon cyclone. Please inform the
Salt Lake Technical Center if you are aware
of another cyclone that matches the characteristics in 29 CFR 1910.1000 Table Z-3.
How do I perform a leak test of the Dorr-Oliver cyclone?
The Cincinnati Technical Center has put together a set of instructions on leak testing
procedures. Click here
for the document.
I need to sample a sand-blasting operation in a room that has no forced ventilation.
The blasting lasts only 20 minutes each day. The employee wears a hood only during the
operation. The employee spends 8 hours a day in the same room with other employees
performing other activities. How long should I sample?
Sample the whole 8-hour work day.
If respirable silica dust is generated during the blasting, it will remain suspended
long afterward. The air is rarely still enough for the dust to completely settle. The
activities of the employee and his/her coworkers would also resuspend dust that has
settled on their clothes. Dust can also be resuspended when workers pass by or over dusty
surfaces, such as the factory floor and when they perform any inappropriate cleanup
operations such as dry sweeping or using an ordinary shopvac. While IHs are often tempted
to sample only during the dust generation operation, doing so will underestimate the
actual exposure – the result of such sampling may easily show up as non-detected
whereas the exposure may be very much larger. For example, if the 20 minute sampling gave
a result just below the detection limit (say, 10 micrograms of quartz) and the air
concentration averaged just half that level throughout the rest of the day, the total
quartz exposure would be about 0.150 mg/m3 – well over the TLV. If this
dust was a typical % quartz composition of say, 20%, the total weight collected over the
day would have been 0.77 mg/m3 giving a severity of exposure of
0.77/[10/(2+20)]=1.7 or 1.7 times the OSHA PEL. The IH should consider such consequences
of short-duration sampling for any air contaminant.
General Information about Silica
The material safety and data sheet (MSDS) for a product consisting of food-grade calcium silicate indicates that
(for example) it contains:
19% CaO
67% SiO2
6 to 8% H2O and
less than 0.1% free silica.
How can that be; isn't SiO2 "free" silica? Should I sample for
quartz?
No and no.
The description indicates that the SiO2 is not "free silica."
"Free silica" includes amorphous silica and the crystalline forms that are not
chemically combined with any other elements. That is to say, silica that is not
"free" is chemically bound in another compound. In this material, the element
silicon is present in the form of silicate compounds and is not in the form of a compound
consisting of SiO2 as implied by the analytical data. The confusion here is due
to the use of the mineralogist's convention in representing the chemical composition of
the product as component oxides. This use is a common and often practical way to report
analytical results and, in this case, does not represent a mixture of the compounds CaO,
SiO2, and H2O. Users of MSDSs should be aware of this common
convention, but the distinction may not be emphasized even in undergraduate chemistry
courses. The material was probably analyzed for Ca, Si, and weight loss on heating. When
the results of the analysis of the product were reported, the gravimetric factors for the
oxides were used with oxygen making up the remainder of the composition. This assumed
oxide composition satisfies the chemical combining capacity of Ca and Si for compounds
formed under normal conditions. The practical value of using oxides can be demonstrated by
looking at the mineralogist's oxide representation of some of the various calcium silicate
compounds that might be present in this product:
CaSiO3 or CaO.SiO2
Ca2SiO4 or 2CaO.SiO2
Ca3SiO5 or 3CaO.SiO2
The downside is that the convention does not always address health concerns.
Note: The term, "free silica" is often used loosely to refer to quartz;
quartz is a "free silica," but not all "free silica" is quartz.
We are removing some old fire brick, fiber glass (or rock wool) heat insulation from
a furnace. Can these materials contain crystalline silica?
The hazards in removal need to be evaluated – if no cutting, breaking,
grinding, etc., is involved there may be no hazard.
In these materials devitrification (conversion of the glassy to the crystalline state)
may have occurred. Devitrification can occur in fire brick, obsidian, pumice, fused
silica, fiber glass, and rock wool held at elevated temperatures producing disordered
crystalline silica forms. These materials should be analyzed for the type and amount of
crystalline silica present before removal so that proper protection can be provided to the
employee. Even without good XRD quantitative confirmation, crystalline silica may be
qualitatively identified.
Just what is "free silica?"
The term, "free silica" is often used loosely to refer to quartz; quartz
is a "free silica," but not all "free silica" is quartz. Amorphous
silica and cristobalite are also examples of "free silica."
Regarding Analytical Procedures
We need OSHA's
detection limit DL for the analysis of crystalline silica in order to apply it to the
results we get from our private contract lab. Where can we obtain OSHA's DL?
As discussed above re. SAEs, contact your contract lab for the detection limit data
they have determined for their analyses.
The qualitative detection limit is usually defined as the amount of analyte equivalent
to three times the standard deviation of the instrument response for low level spikes on
sampling media using the slope of the calibration curve of low level calibration
standards. The low level spikes may be made either at a single low level to obtain the
standard deviation, or the standard error of estimate of the regression for several low
level spikes bracketing the detection limit may be used instead of the standard deviation
at a single level. Ten or more spiked samples are recommended depending on whether spikes
are at a single or several levels.
The quantitative detection limit is usually defined as 3.33 times the qualitative
detection limit defined above.
Can AIHA Proficiency Analytical Testing® (PAT) data for a laboratory from recent
rounds be used to estimate the analytical error portion of the sampling and analysis error
(SAE)?
No.
The PAT program is designed to help consumers select laboratories that are proficient.
In the PAT program analyses of quartz, the "true" values against which a
laboratory's results are compared are based on results from reference laboratories that
are a subset of the participating laboratories. Assuming that the PAT samples were made
from accurately delivered consensus reference material and that the participants all used
the same techniques, instrumentation and methodology, and that the samples are not
otherwise flawed so as to introduce bias, the best accuracy that can be achieved by
consensus analyses is limited by the standard error of the precision of that analysis
[SD/(n)½, where SD is the standard deviation in the results among the n reference labs].
However, the PAT program is not suited to achieve the best accuracy: In the case of
crystalline silica analyses, the analytical equipment and methods vary between labs. X-ray
diffraction, infrared spectroscopy (IR), and colorimetric (chemical) analyses are all in
use. The reference material used in these analyses may also differ in homogeneity and
quality from the National Institute of Standards and Technology (NIST, formerly the NBS)
standard reference material SRM 1878. NIST SRM 1878 is strongly recommended as a consensus
standard for calibrations and tests of analytical accuracy.
The current method of PAT quartz sample generation is by aerosol generation using
"5 micron" Min-U-Sil 5 without cyclones. In addition to any errors in the
generation process, this "total dust" approach introduces a sampling error that
may not duplicate the sampling error associated with the use of a cyclone.
In the PAT program, these generation and sampling errors are recognized as significant
and are evaluated in statistical tests conducted on sub-batches and batches of PAT samples
by the contract laboratory that prepares them. Part of each batch is sacrificed for
characterization. The criterion of acceptability at each concentration level (sub batch)
of PAT quartz samples permits the characterization consisting of 7 samples to have a
maximum CV of 30% at each concentration level of quartz. The corresponding 95% confidence
range would be about 42%. Additionally, a maximum average CV of 20% (for 28 samples) each
set of 4 concentration levels (batch) is permitted. These criteria are currently under
review by the AIHA and NIOSH. The results obtained by participants in the PAT program
therefore include both the analytical error the participating laboratories introduce and
an unknown but potentially large amount of error introduced in the generation and sampling
of the aerosol. These latter errors may vary batch to batch.
Therefore, more appropriate data for monitoring analytical recovery and variability in
the analysis of respirable quartz might be obtained by labs using SRM 1878 (or comparable
reference material) to prepare blind QCs for their in-house quality control program.
Quality control samples are typically prepared from aliquots of well-dispersed suspensions
of the SRM in a solvent or are prepared gravimetrically. NIST prepares its SRM 2679a
Quartz on Filter Media by delivery of a water suspension of SRM 1878 using a thickening
agent to ensure homogenous suspensions. The true value is determined spectroscopically
with a demonstrated precision of 4 to 11% depending on spike level. The deposit is
overlain with a layer of clay. The filter medium is well suited for some IR methods, but
unfortunately does not dissolve in the solvent used for XRD analyses.
Gravimetric QCs may give more accurate delivery and may be prepared by weighing the SRM
or equivalent material onto tared filters or by delivering SRM to a filter as an aerosol
(as in the case of the direct-on-filter methods). (Particle size effects can have opposite
effects on recoveries in XRD and IR. The effect of particle size on the colorimetric
method also strongly depends on analyst technique.)
How can we resolve possible misidentification of opal C or other materials that
mimic cristobalite in the analysis of cristobalite?
One resolution of this problem requires a combined approach using a modified
Talvitie chemical treatment (see description and reference in OSHA ID-142 method). In
order to optimize the conditions for removal of the interferent, recovery tests using
cristobalite-spiked bulk material is needed to ensure that cristobalite is not being
removed along with the opal-C.
This procedure is tedious and is only performed when absolutely necessary. At the OSHA
SLTC, the efficient use of resources is brought about by the procedures for analyzing
compliance air samples for cristobalite given in method ID-142. The type of industrial
operation determines whether cristobalite is analyzed. Air samples suspected of containing
cristobalite are first screened on the most intense X-ray diffraction peak (JCPDS 11-695
an undistorted cristobalite). Based on the screening, if the exposure is above the
detection limit and possibly over a significant fraction of the OSHA permissible exposure
limit (PEL), an attempt is made to confirm on the next 2 most intense peaks. A consensus
Standard Reference Material (SRM) for respirable quartz analyses is currently manufactured
by National Institute of Standards and Technology (NIST, formerly the NBS). The best
confirmation is achieved both quantitatively and qualitatively. We calibrate the
instrument to provide quantitative results on all four quartz diffraction peaks; typically
three are used. The silver membrane used to support the dust is used as an external
2-theta calibration; the peaks should agree within 0.05 degrees 2-theta of the location of
the NIST Standard Reference Material. If the presence of cristobalite is confirmed, a wide
angle scan is performed on the bulk material(s) that may be supplied with the air samples
to look for potential interfering phases. We discuss any unusual situations that arise
with the compliance officer who performed the inspection.
The process I need to sample involves silica (quartz, fused silica, diatomaceous
earth, etc.) that reaches a temperature of only 1100 degrees C – too low for
the formation of cristobalite. Why should I analyze for cristobalite?
Contrary to the usual diagram for the conversion of silica to tridymite (the
diagram shows cristobalite requiring higher temperatures), silica in the temperature
regime of 867 and 1470 degrees C will most often convert to a disordered cristobalite
phase favored by free-energy considerations rather than tridymite. The extent of
disordering depends in part on the presence of trace contaminants and on the thermal
history of the material (sequence and time of heating at the various temperatures
encountered in the process.
XRD is not as sensitive to submicron crystalline silica. Is this an important
consideration?
No. For at least two reasons:
The smallest quartz particles are generally less significant in relation to the total
weight of quartz in a sample. The larger particles in an aerosol generally represent most
of the mass of the aerosol because the mass varies as the cube of the diameter. It takes
125,000 particles, 0.1 micron in size, to equal the mass of a single particle 5 microns in
size.
Due to the presence of an amorphous silica layer on crystalline silica particles, a
smaller proportion of the mass of small particles is crystalline silica. The amorphous
layer is estimated to be approximately, 0.03 micron thick (N.J. Elton, P.D. Salt, and J.M.
Adams, "The Accurate Determination of Quartz in Kaolins by X-ray Powder
Diffraction," Issues and Controversy: The Measurements of Crystalline Silica,
International Symposium, August 20-21, 1992, Cambridge, MA. CMA Crystalline Silica Panel.)
A 0.03-micron layer on a spherical 1-micron diameter quartz particle represents 17% of the
volume of the particle leaving about 83% crystalline. The relative volume of the amorphous
layer increases rapidly with decreasing diameter; a 0.5-micron diameter quartz particle
would be about 68% crystalline, and a 0.1-micron diameter "quartz" particle
would be about 6.4% crystalline. These are high estimates because dusts are rarely
spherical. As a result, the amount of crystalline silica decreases rapidly as particles
become smaller.
|
|

