Physical Activity Guidelines Advisory Committee Report
Part G. Section 8: Mental Health
- Introduction
- Review of the Science
- Overview of Questions
Asked
- Data Sources and Process
Used to Answer Questions
- Question 1: Is There an
Association Between Physical Activity and Depression?
- Question 2. Is There an
Association Between Physical Activity and Anxiety?
- Question 3: Is There an
Association Between Physical Activity and Psychological Distress and
Well-Being?
- Question 4: Is There an
Association Between Physical Activity and Cognitive Function and Dementia?
- Introduction
- Does Physical
Activity Protect Against the Onset of Age-Related Decline in Cognitive Function
or Dementia?
- Does Physical
Activity Reduce Symptoms Associated With Alzheimer’s Disease or Other
Dementias?
- Do the Effects of
Physical Activity on Cognitive Function Differ According to Genetics, Age, Sex,
Race/Ethnicity, or Medical Condition?
- Do the Effects of
Physical Activity Vary According to Features of Physical Activity, Including
Type, Intensity, or Timing (i.e., Session Duration, Weekly Frequency, and
Length of Participation)?
- Question 5: Is There an
Association Between Physical Activity and Sleep?
- Question 6: Is There an
Association Between Physical Activity and Other Aspects of Mental Health?
- Question 7: Is There an
Association Between Physical Activity and Adverse Psychological
Events?
- Question 8: What
Mechanisms Can Plausibly Explain the Association Between Physical Activity and
Mental Health?
- Overall Summary and Conclusions
- Research Needs
- Reference List
List of Figures
Introduction
Poor mental health, including diseases of the central nervous system
(CNS), reduces the quality of life and adds a burden on public health. People
with anxiety or depression disorders are more likely to have chronic physical
conditions (1), and depression and dementia were among the
10 leading risk factors of disability-adjusted life expectancy in high-income
nations worldwide during 2001 (2). They are projected to
rank first and third by the year 2030 (3). In the United
States, dementia and other CNS disorders are a leading cause of death, and
mental disorders are estimated to account for more than 40% of years lost to
disability (4).
The scientific evidence from prospective cohort studies and randomized
controlled trials (RCTs) supports the overall conclusion that regular
participation in moderate-to-vigorous physical activity is associated with
improved aspects of mental well-being and reduced symptoms of several mental
health disorders.
Review of the Science
Overview of Questions Asked
This chapter addresses 7 general questions about physical activity and
mental health conditions. Each question includes subsections that focus on
whether physical activity can protect against the onset of, or reduce,
symptoms; whether the effects of physical activity on the symptoms differ by
age, sex, race/ethnicity, or medical condition; and whether the effects of
physical activity differ by the type, intensity, or timing of the physical
activity. The general questions are:
- Is there an association between physical activity and
depression?
- Is there an association between physical activity and anxiety?
- Is there an association between physical activity and distress and
well-being?
- Is there an association between physical activity and cognitive
function and dementia?
- Is there an association between physical activity and sleep?
- Is there an association between physical activity and other aspects
of mental health?
- Is there an association between physical activity and adverse
psychological events?
The chapter also considers a final question dealing with mechanisms that
could plausibly explain the association between physical activity and mental
health.
Data Sources and Process Used to Answer
Questions
To provide evidence-based answers to the above questions, the Mental
Health subcommittee obtained data from a search of the Physical Activity
Guidelines for Americans Scientific Database (see Part F: Scientific Literature Search
Methodology, for a full description of the Database), which
contains studies published in 1995 and later. Conclusions regarding the
evidence-based review were restricted to results of RCTs and observational
studies that used a prospective cohort design. Findings from selected
cross-sectional, observational studies are presented when they provided
additional information for questions that had a limited number of RCTs and
prospective cohort studies (e.g., anxiety disorders and sleep). Studies of
physical activity and mental health published since 1995 were evaluated using
meta-analytic or otherwise systematic reviews when they were available. When
such reviews were not available or were not sufficiently current, a systematic
quantitative synthesis of results from published studies was conducted using a
random effects model that weights results by the size of each study and
variation in the effects across studies. Odds ratios in observational studies
and standardized effect sizes in RCTs were retrieved from the published papers,
or they were otherwise estimated from data and test statistics reported in the
papers (5).
Question 1: Is There an Association Between
Physical Activity and Depression?
Introduction
The American Psychiatric Association (6) recognizes
4 types of mood disorders: (1) depression, (2) bipolar or
manic-depressive disorder, (3) mood disorders due to a medical
condition, and (4) substance-induced mood disorders. Depression
has an annual prevalence of about 8% among women and 4% among men worldwide and
in the United States. The annual cost of depression in the United States is
estimated at $83 billion per year (7). This condition
includes a mild chronic form, dysthymia, and a more severe form,
major depressive disorder. The rate of major depression has increased
steadily during the past 50 years, with a lifetime prevalence of about 16%; the
rate is higher among Hispanics than whites, and lowest, though still
substantial, among African Americans. The lifetime rate of depression among
adults aged 30 to 60 years is about twice the rate among people older than age
60 years (8).
People have a major depressive episode when they have depressed mood or
lose interest or pleasure in normal activities most of the time for at least 2
weeks. Other symptoms include abnormalities in appetite, libido, sleep, energy
levels, concentration and, often, suicidal thoughts. In some cases, anxiety and
motor agitation can be more prominent symptoms than depressed mood. Also, mood
disturbance can be less apparent than other features such as irritability,
abuse of alcohol, and worsening of comorbid phobias, obsessions, or
preoccupation with physical symptoms. Depression is not considered a major
depressive episode if it is caused by grief (less than 2 months), drug abuse or
medication, or a medical condition such as hyperthyroidism, heart disease,
diabetes, multiple sclerosis, hepatitis, or rheumatoid arthritis. Many older
patients with symptoms of depression do not meet the full criteria for major
depressive disorder. If they have similar, but fewer, symptoms they may have
minor depression, a sub-syndromal form of depression.
Does Physical Activity Protect Against the
Onset of Depression Disorders or Depression Symptoms?
Conclusions
Population-based, prospective cohort studies provide substantial
evidence that regular physical activity protects against the onset of
depression symptoms and major depressive disorder. Evidence is insufficient to
draw conclusions about bipolar disorder and other mood disorders.
Rationale
An association between physical activity and reduced symptoms of
depression among adults has been generally supported in more than 100
population-based observational studies published since 1995, including
nationally representative samples of nearly 190,000 Americans (9-15). Most of the studies looked at cross-sectional
associations, which indicated that active people on average had nearly 45%
lower odds of depression symptoms than did inactive people. In the national
samples of Americans, active people had approximately 30% lower odds of
depression.
Twenty-eight of the studies used a prospective cohort design, which
reduces the likelihood that the association is explainable by people becoming
less active after they experience depression symptoms. The median follow-up was
about 4 years, and the range was 9 months to 37 years. The studies came from 11
nations (Australia, Canada, China, England, Finland, Germany, Israel, Italy,
Netherlands, Japan), including 13 studies from the United States. In the cohort
studies, the average odds of elevated symptoms were about 25 to 40% lower among
active compared with inactive people, without adjustments for depression risk
factors that might have differed between the active and inactive groups
(OR = 0.67, 95% CI = 0.59 to 0.77). After adjustment
for risk factors, such as age, sex, race, education, income, smoking, alcohol
use, chronic health conditions, and other social and psychological variables,
the odds remained nearly 15 to 25 percent lower among active people
(OR = 0.82, 95% CI = 0.78 to 0.86).
Nearly all the comparisons (66 of 67) in the cohort studies showed less
depression among physically active than inactive adults, but half the results
did not reach statistical significance, often because the sample sizes were not
big enough. The average number of people for each comparison was about 1,100
people, but a fourth of the comparisons included 500 people or fewer. Studies
of 8 cohorts (16-23) used a clinical diagnosis to measure
depression symptoms, reporting a reduction in the odds of incident cases of
depression that averaged 30 percent (OR = 0.71, 95% CI: 0.61 to 0.77)
among active people compared to inactive or low active people. In those
studies, 18 of 19 comparisons favored active people, and nearly half the
comparisons were statistically significant. Thus, the protective effect of
physical activity is not limited to self-rated symptoms measured by
questionnaires. Figures G8.1a and G8.1b illustrate crude and adjusted odds
ratios and 95% confidence intervals from the 28 prospective cohort studies
of physical activity and depression in nearly 40,000 adults from 11 countries,
including 11 studies from the United States.
Does Physical Activity Reduce Symptoms of
Depression?
Conclusions
The results of RCTs indicate that participation in physical activity
programs reduces depression symptoms in people diagnosed as depressed, healthy
adults, and medical patients without psychiatric disorders.
Rationale: Depressed Patients
A meta-analysis of 14 RCTs of chronic exercise among people diagnosed
with depression (24) reported a cumulative, mean reduction
in depression symptoms of 1.1 SD (95% CI: 1.5 to 0.60). However, the studies
had scientific weaknesses that made it hard to conclude that the reduced
depression symptoms were the independent result of exercise, and only 2 of the
studies had been published after 1995 (25;26). Since that review, at least 11 RCTs have used exercise
training to reduce depression symptoms in about 500 depressed patients. In
3 studies (27-29), depression was identified using
cut-point scores on symptom questionnaires that have good predictive validity
as screening tests to detect depression. In another 8 studies (30-37), patients were identified by diagnostic interview as
having major depressive disorder, minor depression, or dysthymia.
In the studies published since the Lawlor & Hopker review (24), the average effect of exercise for symptom reduction was
1.1 SD (95% CI: 1.55 to 0.64) compared to a no treatment control group,
virtually identical to the Lawlor & Hopker results. These recent studies
compared the effects of exercise to a placebo (29;32-34;36;38) or to
drug therapy (30;38) or bright light
therapy (35). The reductions, on average, favored exercise
over placebo (0.35 SD, 95% CI: 0.49 to 0.22), and 6 of 14 comparisons were
statistically significant. The average sample size was 58 people, but 25% of
the studies had 25 people or fewer. Symptom reduction after exercise was
similar to drug therapy and similar or superior to bright light therapy. Four
of the studies reported that reductions in symptoms met criteria for a
clinically meaningful decrease in symptoms (50%) or remission (32;35;37;38). Long-term reductions in symptoms were examined in a few
studies and were generally favorable after supervised exercise training ended,
especially when people maintained regular physical activity (28;29;36;39). The trial by Dunn and colleagues. (2005) was a placebo
trial that controlled for social interaction and sunlight exposure (2
confounders common in other studies) and reported response and remission rates
after exercise training that were 40 to 50% better than placebo (32).
Figure G8.1a Depression Symptoms: Prospective
Cohort Studies, 1995 Through 2007: Crude Odds
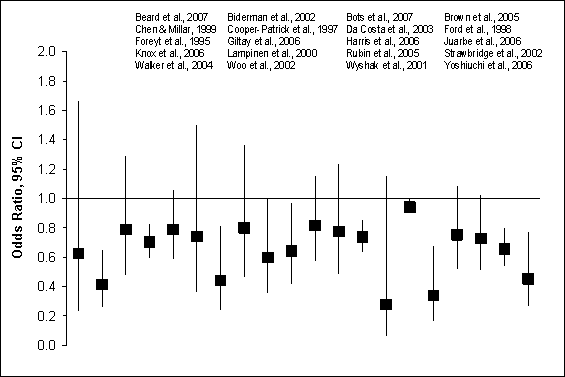
Figure G8.1a Data Points
| Author/Date |
High |
Low |
Odds Ratio |
| Beard et al., 2007 |
1.658 |
0.236 |
0.625 |
| Biderman et al., 2002 |
0.6504 |
0.265 |
0.415 |
| Bots et al., 2007 |
1.289 |
0.4819 |
0.788 |
| Brown et al., 2005 |
0.8251 |
0.6011 |
0.704 |
| Chen & Millar, 1999 |
1.0587 |
0.588 |
0.789 |
| Cooper-Patrick et al., 1997 |
1.5026 |
0.365 |
0.74 |
| Da Costa et al., 2003 |
0.8115 |
0.2407 |
0.442 |
| Ford et al., 1998 |
1.3663 |
0.4686 |
0.8 |
| Foreyt et al., 1995 |
1.0047 |
0.3562 |
0.598 |
| Giltay et al., 2006 |
0.9678 |
0.4232 |
0.64 |
| Harris et al., 2006 |
1.1502 |
0.577 |
0.815 |
| Juarbe et al., 2006 |
1.2356 |
0.4861 |
0.775 |
| Knox et al., 2006 |
0.848 |
0.6394 |
0.736 |
| Lampinen et al., 2000 |
1.1555 |
0.0666 |
0.277 |
| Rubin et al., 2005 |
0.9982 |
0.9229 |
0.94 |
| Strawbridge et al., 2002 |
0.6709 |
0.1698 |
0.338 |
| Walker et al., 2004 |
1.0842 |
0.523 |
0.753 |
| Woo et al., 2002 |
1.0253 |
0.5173 |
0.728 |
| Wyshak et al., 2001 |
0.7946 |
0.5432 |
0.657 |
| Yoshiuchi et al., 2006 |
0.7689 |
0.2658 |
0.452 |
Figure G8.1b Depression Symptoms: Prospective
Cohort Studies, 1995 Through 2007: Adjusted Odds
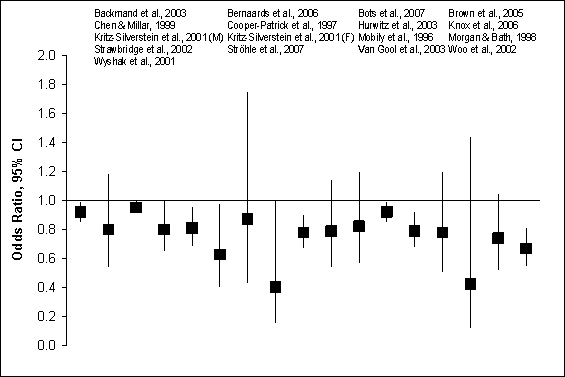
Figure G8.1b Data Points
| Author/Date |
High |
Low |
Odds Ratio |
| Backmand et al., 2003 |
0.9892 |
0.8556 |
0.92 |
| Bernaards et al., 2006 |
1.1774 |
0.5429 |
0.7995 |
| Bots et al., 2007 |
0.9956 |
0.9554 |
0.95 |
| Brown et al., 2005 |
1 |
0.65 |
0.8 |
| Chen & Millar, 1999 |
0.957 |
0.6831 |
0.8086 |
| Cooper-Patrick et al., 1997 |
0.9714 |
0.4021 |
0.625 |
| Hurwitz et al., 2003 |
1.7464 |
0.4326 |
0.8692 |
| Knox et al., 2006 |
0.999 |
0.1602 |
0.4 |
| Kritz-Silverstein et al., 2001(M) |
0.8946 |
0.6746 |
0.7769 |
| Kritz-Silverstein et al., 2001 (F) |
1.141 |
0.5439 |
0.7878 |
| Mobily et al., 1996 |
1.1928 |
0.5659 |
0.8216 |
| Morgan & Bath, 1998 |
0.99 |
0.855 |
0.92 |
| Strawbridge et al., 2002 |
0.9205 |
0.678 |
0.79 |
| Ströhle et al., 2007 |
1.1945 |
0.5072 |
0.7784 |
| Van Gool et al., 2003 |
1.4337 |
0.1242 |
0.422 |
| Woo et al., 2002 |
1.0435 |
0.5224 |
0.7383 |
| Wyshak et al., 2001 |
0.8114 |
0.5472 |
0.6663 |
Rationale: Healthy Adults and Adults With Medical Conditions
At least 42 RCTs since 1995, including more than 2,600 people, have
examined the effects of exercise on depression symptoms in healthy adults or
adults with medical conditions other than depression or disabling conditions
that severely limit physical activity (i.e., spinal cord injury, multiple
sclerosis, and stroke or traumatic head injury). Most of the medical patients
studied had cardiovascular diseases, arthritis, chronic pain, obesity, or were
cancer survivors. The mean effect of exercise compared to a control condition
was 0.35 SD (95% CI: 0.24 to 0.46). The outcomes favored exercise in 90%
(34 of 38) of the comparisons with control conditions, of which 60% (23 of 38)
reached statistical significance. The average sample size was about 65 people,
but in about a fourth of the studies it was less than 35, which was too small
to detect an effect smaller than a half standard deviation (SD). When compared
to a placebo (usually stretching or health education), the effect of exercise
was halved to 0.15 SD (95% CI: 0.24 to 0.06). Although exercise effects
exceeded placebo effects in 19 of 22 comparisons, only 4 reached statistical
significance. The average sample size in the placebo studies was nearly 100
people, but samples more than 4 times that size would be needed to detect an
effect that small.
Do the Effects of Physical Activity on
Depression Symptoms Differ According to Age, Sex, Race/Ethnicity, or Medical
Condition?
Conclusions
The available evidence supports the conclusion that regular physical
activity reduces depression symptoms regardless of age, sex, race/ethnicity, or
medical condition. Whether these factors modify the association between
physical activity and depression has been understudied. Also, race and
ethnicity have been poorly represented or not described in most studies.
Rationale: Cohort Studies
Few prospective cohort studies reported findings according to sex or age
groups. Just 1 study reported results for men and women, finding that
lower levels of depression symptoms were associated with more physical activity
in both men and women (40). Only 11 studies had separate
results for men or women. Reductions were smaller for active men
(OR = 0.96, 95% CI = 0.93 to 0.99) than women
(OR = 0.72, 95% CI = 0.68 to 0.77), but men were less
studied. Five studies totaled fewer than 4,000 men, while 6 studies totaled
more than 14,000 women. After adjusting for the number of comparisons made in
each of the other cohort studies, in which men and women were represented
evenly, effects for men and women did not differ. Crude odds reduction among
active people were greater for people aged 55 years or older
(OR = 0.58; 95% CI = 0.46 to 0.72) than people younger than
age 55 year (OR = 0.72; 95% CI = 0.64 to 0.81), but
physical activity was protective for all age groups, regardless of sex.
Most of the prospective cohort studies were population-based, but only a
third specified the proportions of racial and ethnic groups included, and only
4 studies had evidence of good representation of African Americans and/or
Hispanics/Latinos (41-44). One study reported that the
odds of depression symptoms were similarly lower among white and African
American adults who were active (42). Other studies had
poor or no representation of other minority groups.
Rationale: Randomized Controlled Trials
In RCTs of people without depression, the average effect of exercise
compared to a control condition is 0.35 SD in people with medical conditions
(24 studies; 95% CI: 0.47 to 0.23) and 0.35 SD in people who have not been
diagnosed with a medical condition (7 studies; 95% CI: 0.59 to 0.11).
Figure G8.2 illustrates effect sizes and 95% confidence intervals comparing
exercise with a control condition from the RCTs of depression in adults with or
without medical conditions.
These trials reported similar reductions in depression for groups of
varying ages from 28 to 83 years, but the studies were not designed to compare
groups according to sex, race/ethnicity, or age. Only 1 of the trials compared
men and women (45) and none compared effects between
people of different ages or race/ethnicities. Seven of 11 studies of Americans
described the racial/ethnic composition of their samples, but only 5 studies
included African Americans (46-50); 3 studies included
small numbers of Hispanics, Asian Americans or American Indians (49-51). Effects were larger in 6 studies of men
(mean = 0.86 SD, 95% CI = 0.45 to 1.26), compared to 9
studies of women (mean = 0.27 SD, 95% CI = 0.04 to 0.51)
and to 23 studies that combined results of men and women (mean = 0.27
SD, 95% CI = 0.16 to 0.37). Thus, it is likely that other features of
the studies of men might explain the larger effects.
Reductions in depression symptoms were larger in 8 studies of heart
patients (mean = 0.60 SD, 95% CI = 0.33 to 0.86) than in
studies of other medical conditions (mean = 0.23 SD, 95%
CI = 0.11 to 0.34) and were largest in the 3 studies of heart
patients that included only men (52-54)
(mean = 1.0 SD, 95% CI = 0.50 to 1.5). The small number of
studies precluded meaningful comparisons among the other medical
conditions.
Figure G8.2 Depression Symptoms: Randomized
Controlled Trials, 1995 Through 2007

Figure G8.2. Data Points
| Healthy Adults |
High |
Low |
Effect Size |
| Antunes et al., 2005 |
1.5775 |
0.3545 |
0.966 |
| Atlantis et al., 2004 |
0.8808 |
−0.3108 |
0.285 |
| Chin A Paw et al., 2004 |
0.4298 |
−0.4718 |
−0.021 |
| Damush et al., 1999 |
0.6628 |
−0.3368 |
0.163 |
| DiLorenzo et al., 2004 |
1.2781 |
0.4039 |
0.841 |
| Li et al., 2001 |
0.7952 |
−0.1406 |
0.3273 |
| Sørensen et al., 1999 |
0.4545 |
−0.3687 |
0.0429 |
| Medical Patients |
High |
Low |
Effect Size |
| Anderson et al., 2005 |
0.3863 |
−0.1703 |
0.108 |
| Blumenthal et al. 2005 |
0.7735 |
−0.0615 |
0.356 |
| Clark et al., 2000 |
0.6815 |
−0.5729 |
0.0543 |
| Courneya et al., 2003 |
0.4774 |
−0.3524 |
0.0625 |
| Daley et al., 2007 |
1.2058 |
0.2102 |
0.708 |
| Dugmore et al., 1999 |
1.4473 |
0.6947 |
1.071 |
| Emery et al., 1998 |
1.1587 |
0.0003 |
0.5795 |
| Gary & Lee, 2007 |
2.0279 |
0.2521 |
1.14 |
| Gowans et al., 2001 |
1.2908 |
−0.1478 |
0.5715 |
| Kiernan et al., 2001 |
0.4897 |
−0.4197 |
0.035 |
| Koukouvou et al., 2004 |
2.3344 |
0.5646 |
1.4495 |
| Mannerkorpi et al., 2000 |
0.7909 |
−0.2519 |
0.2695 |
| Neidig et al., 2003 |
0.97 |
−0.059 |
0.4555 |
| Neuberger et al., 2007 |
0.2436 |
−0.3876 |
−0.072 |
| O’Reilly et al., 1999 |
0.5329 |
−0.0669 |
0.233 |
| Paz-Diaz et al., 2006 |
1.561 |
−0.1128 |
0.7241 |
| Rolland et al., 2007 |
0.4932 |
−0.185 |
0.1541 |
| Schachter et al., 2003 |
0.8583 |
−0.1649 |
0.3467 |
| Scholz et al., 2006 |
0.7897 |
0.2233 |
0.5065 |
| Segar et al., 1998 |
2.1691 |
−0.1017 |
1.0337 |
| Seki et al., 2003 |
0.935 |
−0.3452 |
0.2949 |
| Tench et al., 2003 |
0.6196 |
−0.3542 |
0.1327 |
| Van den Berg-Emons et al., 2004 |
1.0801 |
−0.2801 |
0.4 |
| Yu et al., 2004 |
0.4876 |
−0.0886 |
0.1995 |
Do the Effects of Physical Activity Vary
According to Features of Physical Activity, Including Type, Intensity, or
Timing (i.e., Session Duration, Weekly Frequency, and Length of
Participation)?
Conclusions
The evidence from prospective cohort studies and RCTs published since
1995 suggests that moderate and high levels of physical activity similarly
reduce the odds of developing depression symptoms compared to low levels of
physical activity exposure, which is nonetheless more protective than
inactivity or very low levels of physical activity. The minimal or optimal type
or amount of exercise for reducing depression symptoms is not yet known, but it
appears that an increase in physical fitness is not required.
Rationale
Epidemiologic studies typically use a variety of criteria and methods to
classify people into 2 or more activity groups. This limits the evaluation of
the dose-response relation across the full range of reported physical activity
and can misclassify people who overestimate or underestimate their activity.
Only 7 prospective cohort studies of depression symptoms included the 3 or more
levels of physical activity necessary to determine whether the association of
physical activity with lower odds of depression has a dose-gradient with
increased levels of exposure (16;18;21;22;55-57). After adjustment for age, sex, and other risk
factors, the reduction of odds was smaller for the lowest level of physical
activity (OR = 0.86, 95% CI = 0.79 to 0.94) compared to the
next 2 levels of physical activity, which did not differ (OR = 0.77,
95% CI = 0.72 to 0.82). Thus, the highest levels of participation did
not confer more protective benefits than did more moderate levels. Each was
more protective than the lowest levels in the studies.
Figure G8.3 shows the odds ratios and 95% CI in prospective cohort
studies that examined the dose-response association between levels of physical
activity and depression symptoms.
Those studies used different measures and criteria for defining levels
of physical activity, so it is not possible to convert their findings to a
standard estimate (e.g., MET-hours or kilocalories per kilogram) of the amount
of physical activity at each level. However, about half the prospective cohort
studies provided enough information to determine whether active people were
meeting existing public health recommendations (58;59) for participation in moderate or
vigorous physical activity (i.e., moderate-intensity aerobic [endurance]
physical activity for a minimum of 30 minutes on 5 days per week, or
vigorous-intensity aerobic physical activity for a minimum of 20 minutes on 3
days per week). Odds reduction favored people who met or exceeded
recommendations for moderate or vigorous participation (OR = 0.64,
95% CI = 0.57 to 0.73) compared to active people who did not meet
either recommended level (OR = 0.70, 95% CI = 0.58 to
0.82). Odds were not different between vigorous and moderate participation, but
it was possible to distinguish moderate from vigorous participation in only a
few studies. After adjustment for other risk factors, a similar protective
benefit favored regular participation in moderate or vigorous physical activity
(OR = 0.77, 95% CI = 0.72 to 0.82) compared to
participation at levels less than recommended (OR = 0.84, 95%
CI = 0.78 to 0.90).
Figure G8.3 Depression Symptoms: Prospective
Cohort Studies 1995 Through 2007: Dose Response
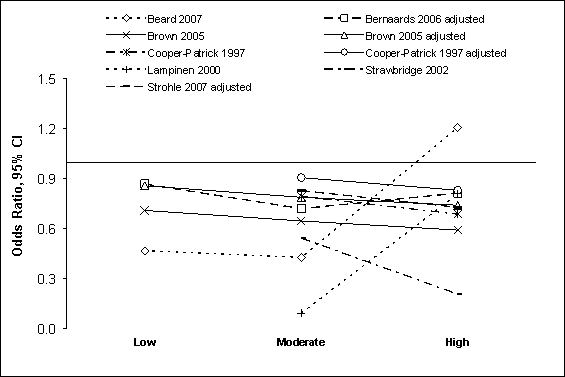
Figure G8.3 Data Points
| Author/Year |
Low |
Moderate |
High |
| Beard 2007 |
0.47 |
0.43 |
1.21 |
| Bernaards 2006 adjusted |
0.8694 |
0.7218 |
0.8151 |
| Brown 2005 |
0.711 |
0.648 |
0.593 |
| Brown 2005 adjusted |
0.859 |
0.79 |
0.742 |
| Cooper-Patrick 1997 |
0.796 |
0.689 |
|
| Cooper-Patrick 1997 adjusted |
0.908 |
0.832 |
|
| Lampinen 2000 |
0.095 |
0.81 |
|
| Strawbridge 2002 |
|
0.545 |
0.209 |
| Strohle 2007 adjusted |
0.8303 |
0.7298 |
|
A few RCTs of people without depressive disorders have manipulated type
(e.g., aerobic versus resistance or walking versus aquatic exercise or Qigong)
or timing (i.e., continuous versus intermittent [the intermittent studies often
used resistance, circuit, interval or mixed-mode exercise]), of exercise to
examine whether those features modify the effects of exercise on symptoms of
depression (60-64). However, these studies did not include
a control group who did not exercise. Only a few studies have evaluated Eastern
health practices that include exercise (65). About 40
percent of the studies used an aerobic exercise intervention such as walking,
jogging, cycling, or aquatic exercise, and another third of the studies
combined aerobic activity with resistance exercise. Only 3 studies used
resistance exercise alone, and none compared aerobic versus resistance
exercise. On average, reductions in depression have been similar regardless of
the mode of activity used. About two-thirds of the studies used continuous
exercise. Regardless of mode, a larger reduction in depression tends to occur
after continuous exercise (0.45 SD, 95% CI = 0.63 to 0.27) than
intermittent exercise (0.18 SD, 95% CI = 0.30 to 0.06), but differing
features of the studies other than timing might explain this finding.
Three-fourths of the RCTs of healthy adults and non-psychiatric medical
patients used a moderate-to-vigorous exercise intensity of 60-80% of
people’s aerobic capacity or maximum strength that occurred 3 days per
week. Intensity was lower or the frequency was 2 days per week in the other
studies. The average duration of each session was about 35 minutes, but it
was less than 30 minutes in a fourth of the studies and more than 1 hour in
another fourth of the studies. However, fewer than half the studies were clear
about how the time was partitioned into warm-up, exercise, and cool down.
Nonetheless, reductions in depression symptoms did not differ across these
varying features of exercise. Studies lasted an average of 6 months, and a
fourth of the studies lasted less than 3 months. Length of the exercise program
also was unrelated to symptom outcome. About half the studies measured
cardiorespiratory fitness, which was increased significantly in 16 of 24
comparisons. Only 3 studies measured strength. In each study, fitness
increases and symptom reduction were not associated when changes were defined
by statistical significance, which depends on both the size of change and the
sample size. After adjusting for sample sizes, the magnitude of depression
reduction was moderately correlated with the magnitude of fitness increase
(r = 0.40). However, that relation was not independent of increases
in primary outcomes other than fitness in the studies of medical patients.
Perhaps the clearest experimental evidence for a dose-dependent effect
of exercise on symptom reduction comes from the dose study (32). Adults aged 20 to 45 years diagnosed with mild to
moderate major depressive disorder expended either 7.0 or 17.5 kilocalories per
kilogram per week at a frequency of 3 or 5 days per week or engaged in 3 days
per week of stretching exercises as a placebo control. Physician-rated symptoms
after 12 weeks were reduced 47% from baseline for the higher dose, compared
with 30% for the lower dose and 29% for control, regardless of whether exercise
frequency was 3 days or 5 days each week.
Question 2. Is There an Association Between
Physical Activity and Anxiety?
Introduction
Anxiety is characterized by apprehensive or worrisome thoughts and is
typically accompanied by agitation, feelings of tension, and activation of the
autonomic nervous system. A distinction is made between transient anxiety
symptoms, termed state anxiety, persistent symptoms, termed trait
anxiety, and a group of disabling conditions characterized by excessive,
chronic anxiety that are known as anxiety disorders. The anxiety
disorders, listed from most to least common, are:
- Specific phobia — an intense fear of an object, place,
or situation that poses little or no actual danger.
- Social phobia — an overwhelming fear of scrutiny and
embarrassment in social situations, leading to avoidance of potentially
enjoyable activities.
- Generalized anxiety disorder — recurrent or persistent
excessive worry about everyday, routine life events and activities, lasting at
least 6 months.
- Panic disorder — repeated episodes of intense fear and
physical symptoms that strike without warning and without an obvious source,
often producing fear of being alone or going into public places
(agoraphobia) and persistent fear of an attack.
- Obsessive-compulsive disorder — repeated, unwanted
thoughts or compulsive behaviors that seem impossible to stop, typified by
repetitive acts or rituals to relieve anxiety.
- Post–traumatic stress disorder — a delayed or
prolonged response (including flashbacks, dreams, insomnia, hypervigilance) to
a stressful event or situation (either short- or long-lasting) that was
especially threatening or catastrophic (6).
Anxiety disorders are common, affecting more than 16 million people in
the United States each year (roughly 4% of women and 2% of men). More than 80
million people in the United States at some point in their lives suffer from an
anxiety disorder (8). Anxiety disorders begin at a median
age of 15 years, often persist throughout life and are associated with numerous
physical and mental co-morbidities, especially depression (66). People aged 15 to 24 years experience episodes of
anxiety about 40% more often than people aged 25 to 54 years, regardless of
race. Although less than 30% of those who suffer from anxiety disorders seek
treatment, they strain the health care system because of direct psychiatric and
nonpsychiatric treatment costs. Additional indirect costs of anxiety disorders
are incurred from reduced work productivity. The total annual costs in 1990 of
all anxiety disorders were estimated to be $42 billion to $47 billion dollars
(67;68), and they likely cost double
that amount today.
Does Physical Activity Protect Against the
Onset of Anxiety Disorders or Anxiety Symptoms?
Conclusions
The weight of evidence from a small number of nationally representative
and population-based cross-sectional and prospective cohort studies supports
that regular physical activity protects against the onset of anxiety disorders
and anxiety symptoms.
Rationale
At least 4 population-based cross sectional studies published since
1995, including data from nationally representative samples of nearly 121,000
Americans, show that regular physical activity is associated with lower odds of
anxiety symptoms (13;69-71). Results
of the US National Co-Morbidity Study found that regular physical activity
reduced the odds of a diagnosed anxiety disorder (i.e., specific phobia, social
phobia, generalized anxiety, panic, and agoraphobia) by an average of 43% (10). After controlling for sociodemographic and illness
variables, regular physical activity reduced the odds of an anxiety disorder by
an average of 28% (10).
At least 2 population-based studies used a prospective cohort design.
The odds of developing any anxiety disorder were reduced by an average of 53%
among Australians who reported more than 3 hours per week of vigorous physical
activity compared to those reporting no activity (16). The
effect was not statistically significant, in part due to the small number of
participants who developed an anxiety disorder (n=67). The second study, which
adjusted for age and sex and had more participants who developed an anxiety
disorder (n=228), found that regularly active young adults (representative of
Munich, Germany) on average had a statistically significant, 48% lower odds of
developing any anxiety disorder compared to those reporting no activity (22).
Does Physical Activity Reduce Anxiety
Symptoms?
Conclusions
The results of RCTs conducted with medical patients and healthy adults
indicate that participation in physical activity programs reduces anxiety
symptoms.
Rationale
Before 1995, no reports of RCTs of anxiety disorder patients had been
reported. Since then, 2 RCTs have been conducted with anxiety disorder patients
and reported statistically large effects. One trial conducted with 46 panic
disorder patients found that those who completed a 10-week (3 times per week)
walking or jogging program reported, on average, a large reduction in anxiety
symptoms (1.1 SD) compared to those who took daily placebo capsules (72). The other trial involved 74 patients with social phobia,
generalized anxiety, or panic disorder. The addition of a moderate intensity
home-based exercise program to 8 to 10 weeks of group cognitive-behavioral
therapy (GCBT) resulted in a large reduction in anxiety symptoms (1.36 SD)
compared to the control condition (GCBT + nutrition education) after
statistically controlling for potential confounding variables (73).
Before 1995, at least 40 quasi-experimental and experimental exercise
training studies of varying quality had reported a cumulative effect on
reducing anxiety symptoms of approximately 0.40 SD (74).
Since 1995, at least 46 RCTs involving more than 3,550 people have examined the
effects of chronic exercise on anxiety symptoms among inactive adults who were
healthy or had a medical condition other than anxiety disorders or disabling
CNS disorders including multiple sclerosis, traumatic head injury, stroke or
spinal cord injury. The effect of exercise compared to control conditions in
reducing anxiety symptoms was 0.38 SD (95% CI: 0.30 to 0.46). Outcomes favored
exercise in 84% (67 of 80) of the comparisons with control conditions, and 28%
(22 of 80) reached statistical significance. In 6 studies that compared
moderate-to-vigorous exercise to a placebo-type condition (usually low
intensity exercise such as stretching) the effect of moderate-to-vigorous
exercise on reducing anxiety symptoms was 0.19 SD (95% CI: 0.05 to 0.33).
Exercise effects were favorable in 90% (9 of 10) of the placebo-type
comparisons, and 30% reached statistical significance. The average study
included about 60 people, but a fourth of the studies had less than 40
participants, too few to detect small but possibly meaningful effects.
Do the Effects of Physical Activity on
Anxiety Symptoms Differ According to Age, Sex, Race/Ethnicity, or Medical
Condition?
Conclusions
The weight of the evidence supports the conclusion that regular physical
activity reduces anxiety symptoms regardless of age, sex or medical condition.
No published data address whether race or ethnicity modifies the effects of
physical activity on anxiety symptoms.
Rationale
In the 46 RCTs of healthy adults and medical patients, the mean age of
the samples was weakly related to anxiety reductions after exercise training.
Two of the trials compared men and women and found no sex-related difference in
the effect of exercise on anxiety symptoms (45;75). The average effect of exercise training on reduced
symptoms of anxiety was larger in those studies that involved only men
(mean = 0.62 SD, 95% CI: 0.34 to 0.89, n=7) compared to those that
included only women (mean = 0.33 SD, 95% CI: 0.16 to 0.50, n=12) and
those that included similar proportions of men and women (mean = 0.42
SD, 95% CI: 0.28 to 0.57, n=19). It is likely that other features of the
studies of men might explain the larger effects. Regardless, exercise benefited
both men and women.
None of the available investigations was designed to determine whether
the effect of chronic exercise was moderated by race or ethnicity. The racial
or ethnic composition of the samples was described in 17% of the reviewed
studies (25;51;76-81). Of the 3,550 people who participated in the RCTs,
approximately 89% were whites and approximately 11% were African Americans or
Hispanics. Statistical analyses by race or ethnic category were not presented
in any of the studies.
Of the RCTs, about 40% (19 of 46) were conducted with healthy adults,
and 60% (27 of 46) involved patients with various medical conditions. The most
frequently studied conditions were cancer and cardiovascular diseases. The
average effect of exercise compared to a control condition was 0.40 SD in
healthy adults (40 comparisons; 95% CI: 0.27 to 0.53) and 0.36 in people with
medical conditions (40 comparisons; 95% CI: 0.26 to 0.47). The reduction in
anxiety symptoms was somewhat larger than average in studies of cardiovascular
conditions (mean = 0.53 SD, 95% CI: 0.15 to 0.92, n=6), but too few
studies are available to conclude that the effect of exercise training on
anxiety symptoms is modified by medical condition. Figures G8.4a and G8.4b
summarize the findings from the RCTs of physical activity and anxiety symptoms
in healthy adults and medical patients.
Epidemiologic studies have not yet examined race/ethnicity or medical
condition as potential modifiers of the effect of exercise on anxiety disorders
or symptoms. One cross-sectional study of 41,914 participants in the 2001
Behavioral Risk Factor Surveillance System found low anxiety symptoms among
physically active people compared to inactive people across young (age 18 to 40
years), middle-aged (aged 41 to 60 years) and older adult (aged 61 years and
older) categories, but the inactive younger adults were approximately 20% more
likely to experience anxiety symptoms than inactive middle-aged and older
adults (13). In a population-based cross sectional study
of 19,288 twins and their families, potential interactions of exercise and age,
exercise and sex and exercise, age and sex on anxiety symptoms were
statistically non-significant (69).
Do the Effects of Physical Activity Vary
According to Features of Physical Activity Including Type, Intensity, or Timing
(i.e., Session Duration, Weekly Frequency, and Length of Participation)?
Conclusions
Limited cross-sectional, observational evidence suggests that the odds
of an anxiety disorder may be reduced by higher weekly frequency of exercise
bouts. However, there is an absence of evidence from prospective cohort studies
or RCTs that examine whether anxiety symptoms vary according to features of
physical activity exposure.
Rationale
One large population-based study found a dose-response relation between
cross-sectional measures of physical activity frequency and lower prevalence of
anxiety disorders (i.e., specific phobia, social phobia, generalized anxiety,
panic, and agoraphobia) (10). The percentage of adults
with these anxiety disorders was highest among those who reported no physical
activity and was reduced in a step-wise fashion among those who reported rare,
occasional, and regular physical activity (10).
Figure G8.4a Anxiety Symptoms: Randomized
Controlled Trials of Healthy Adults 1995 Through 2007
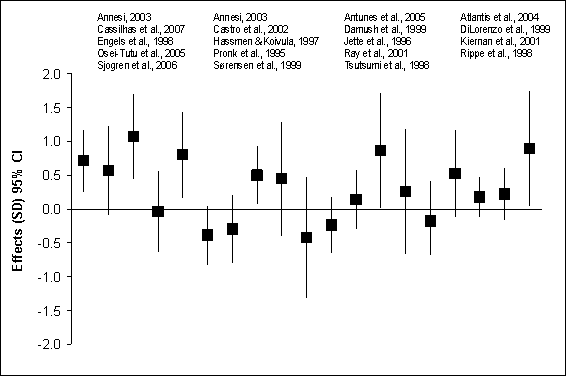
Figure G8.4a Data Points
| Author |
Year |
High |
Low |
Effect Size |
| Annesi |
2003 |
1.1726 |
0.2534 |
0.713 |
| Annesi |
2003 |
1.2184 |
−0.0811 |
0.5687 |
| Antunes et al. |
2005 |
1.6922 |
0.4556 |
1.0739 |
| Atlantis et al. |
2004 |
0.5596 |
−0.6273 |
−0.0339 |
| Cassilhas et al. |
2007 |
1.4338 |
0.1748 |
0.8043 |
| Castro et al. |
2002 |
0.047 |
−0.8126 |
−0.3828 |
| Damush et al. |
1999 |
0.2104 |
−0.7926 |
−0.2911 |
| DiLorenzo et al. |
1999 |
0.9291 |
0.072 |
0.5006 |
| Engels et al. |
1998 |
1.2926 |
−0.3902 |
0.4512 |
| Hassmen & Koivula |
1997 |
0.4711 |
−1.3073 |
−0.4181 |
| Jette et al. |
1996 |
0.1764 |
−0.6431 |
−0.2334 |
| Kiernan et al. |
2001 |
0.5843 |
−0.2918 |
0.1463 |
| Osei-Tutu et al. |
2005 |
1.7087 |
0.0233 |
0.866 |
| Pronk et al. |
1995 |
1.1766 |
−0.6525 |
0.262 |
| Ray et al. |
2001 |
0.4161 |
−0.6794 |
−0.1767 |
| Rippe et al. |
1998 |
1.1732 |
−0.1149 |
0.5292 |
| Sjogren et al. |
2006 |
0.472 |
−0.1135 |
0.1792 |
| Sǿrensen et al. |
1999 |
0.6008 |
−0.159 |
0.2209 |
| Tsutsumi et al. |
1998 |
1.7363 |
0.052 |
0.8946 |
Figure G8.4b. Anxiety Symptoms: Randomized
Controlled Trials of Medical Patients 1995 Through 2007
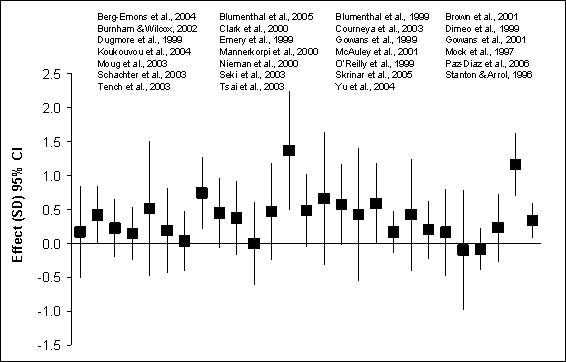
Figure G8.4b Data Points
| Author |
Year |
High |
Low |
Effect Size |
| Berg-Emons et al. |
2004 |
0.8471 |
−0.5021 |
0.1725 |
| Blumenthal et al. |
2005 |
0.8372 |
0 |
0.4186 |
| Blumenthal et al. |
1999 |
0.6547 |
−0.1989 |
0.2279 |
| Brown et al. |
2001 |
0.532 |
−0.2394 |
0.1463 |
| Burnham & Wilcox. |
2002 |
1.5144 |
−0.4748 |
0.5198 |
| Clark et al. |
2003 |
0.812 |
−0.4264 |
0.1928 |
| Courneya et al. |
2000 |
0.471 |
−0.3913 |
0.0399 |
| Dimeo et al. |
1999 |
1.2758 |
0.2166 |
0.7462 |
| Dugmore et al. |
1999 |
0.9564 |
−0.0625 |
0.447 |
| Emery et al. |
1999 |
0.9157 |
−0.1661 |
0.3748 |
| Gowans et al. |
1999 |
0.6123 |
−0.6124 |
0 |
| Gowans et al. |
2001 |
1.1875 |
−0.2423 |
0.4726 |
| Koukouvou et al. |
2004 |
2.2472 |
0.4995 |
1.3733 |
| Mannerkorpi et al. |
2000 |
1.0158 |
−0.039 |
0.4884 |
| McAuley et al. |
2001 |
1.6432 |
−0.3133 |
0.665 |
| Mock et al. |
1997 |
1.1626 |
−0.0178 |
0.5724 |
| Moug et al. |
2003 |
1.3995 |
−0.5531 |
0.4232 |
| Nieman et al. |
2000 |
1.1882 |
−0.004 |
0.5921 |
| O'Reilly et al. |
1999 |
0.4695 |
−0.1279 |
0.1708 |
| Paz-Diaz et al. |
2006 |
1.25 |
−0.3972 |
0.4264 |
| Schachter et al. |
2003 |
0.6268 |
−0.2207 |
0.2031 |
| Seki et al. |
2003 |
0.8051 |
−0.4711 |
0.167 |
| Skrinar et al. |
2005 |
0.7841 |
−0.9789 |
−0.0974 |
| Stanton & Arrol. |
1996 |
0.2212 |
−0.3778 |
−0.0829 |
| Tench et al. |
2003 |
0.7356 |
−0.2636 |
0.236 |
| Tsai et al. |
2003 |
1.6238 |
0.7053 |
1.1645 |
| Yu et al. |
2004 |
0.5979 |
0.0852 |
0.3415 |
Prospective cohort studies show mixed results as to whether
participation in sports, activities that often involve high-intensity exercise,
has a protective effect against anxiety symptoms. Some show a protective effect
(23) and others show no protection (82). One nationally representative cross-sectional study
found that a group of regularly physically active (i.e., met or exceeded the
Healthy People 2010 physical activity guidelines) people reported a
higher frequency of anxiety symptoms compared to those who were physically
active but not active enough to meet the Healthy People 2010
recommendations (13).
Few RCTs of healthy adults or people with medical conditions have
manipulated any feature of an exercise intervention to learn whether those
features causally modify anxiety symptoms. No exercise training studies have
manipulated exercise program length or exercise type for the purpose of
examining anxiety symptom outcomes.
About 43% (20 of 46) of the studies used a single aerobic exercise
intervention such as walking, jogging, or cycling, 13% (6 of 46) used
resistance exercise alone, and 9% (4 of 46) combined aerobic with resistance
exercise. The magnitude of anxiety reduction has been similar across these 3
categories of studies. Regardless of mode, anxiety reductions also were similar
after continuous (0.36 SD, 95% CI: 0.27 to 0.45) and intermittent (0.39 SD, 95%
CI: 0.16 to 0.63) exercise (about 60% of studies used continuous exercise).
Three investigations manipulated exercise session duration and found no
significant differences in anxiety symptom outcomes among exercise training
studies that used bouts of varying session durations (80;83;84). Across
all trials, the average duration of the exercise sessions was approximately 40
minutes. In a fourth of the studies, the duration was less than 25 minutes, and
in another fourth the duration was more than 60 minutes. The effects of
exercise training on anxiety symptoms were similar across studies of all
durations. Imprecise descriptions of exercise duration contributed to the
difficulty in determining whether session duration has a true effect. For
example, less than half of the studies were specific regarding how time was
partitioned into warm-up, exercise, and cool down.
Only 2 exercise training experiments manipulated exercise intensity to
examine whether it modifies the effects of exercise on anxiety symptoms.
Statistically non-significant differences in anxiety symptoms were found
between moderate- and high-intensity resistance exercise conditions on the
anxiety symptom outcomes (81;85).
About 55% (44 of 80) of the comparisons from all the trials used
moderate-to-vigorous exercise intensity (i.e., 60 – 80% of
aerobic capacity or maximum strength) with a weekly frequency of 3 or more days
per week. Reductions in anxiety symptoms were similar across variations in
exercise intensity.
About 63% (29 of 46) of the trials measured fitness, 7 studies measured
strength, and 22 studies measured cardiorespiratory fitness.
Cardiorespiratory fitness was increased significantly in 36% (13 of 36) of the
comparisons and strength was increased significantly in 25% (2 of 8) of the
comparisons. Those studies that defined changes by statistical significance
showed no association between fitness increases and anxiety symptom reduction.
After adjusting for sample sizes, the magnitude of anxiety reduction was weakly
correlated with the magnitude of fitness increase (r = .24). This
relation was independent of increases in primary outcomes other than fitness in
the studies.
Question 3: Is There an Association Between
Physical Activity and Psychological Distress and Well-Being?
Introduction
Psychological distress is a risk factor for psychiatric disorders (86;87) and coronary heart disease (88), and it is negatively associated with quality of life.
Conversely, a feeling of well-being can reduce psychiatric risk and is an
important feature of high life quality and health (89).
People frequently experience feelings of distress during the normal course of
living and during challenging life events, including chronic medical
conditions. Thus, it is important to understand the association between
physical activity and feelings of distress or well-being because they bear not
only on disease risk but also on overall mental health. Measures in this area
are not uniform, but most studies have used a scale that assessed the presence
of distress (e.g., combined symptoms of anxiety and depression or perceived
stress) or the absence of distress (e.g., well-being or positive mental
health). Findings of physical activity studies have not differed when measures
of distress or well-being were used, so the following results apply regardless
of the direction of odds (i.e., decrease in distress or increase in
well-being).
Does Physical Activity Protect Against the
Onset of Feelings of Distress or Enhance Well-Being?
Conclusions
The available evidence from prospective cohort studies indicates a
small-to-moderate association that favors people who are physically active.
Rationale
The association between physical activity and reduced feelings of
distress or enhanced well-being among adults was virtually unstudied in large
groups of people before 1995 (90). Since then, more than
30 population-based observational studies have been published, including
nationally representative samples of more than 175,000 Americans (91-95). Most of the studies looked at cross-sectional
associations, which indicated that active people on average had more than a 30%
lower odds of feeling of distress or 30% higher odds of enhanced well-being
than did inactive people. In the national samples of Americans, the odds
favored active people by approximately 25%.
Thirteen studies of adults in Australia, Canada, Denmark, England,
Netherlands, Scotland, Wales, and 3 studies of Americans (18;23;94) used a
prospective cohort design. In those studies, the average odds of reduced
feelings of distress or of enhanced well-being favored active people by about
30% compared with inactive people, without adjustments for risk factors
(OR = 0.69, 95% CI = 0.61 to 0.78). After adjustment for
risk factors, such as age, sex, race, education, social class, occupation,
income, smoking, alcohol use, substance abuse, chronic health conditions,
disability, marital status, life events, job stress, and social support, the
odds still favored active people by nearly 20% (OR = 0.82, 95%
CI = 0.77 to 0.86). About 80% of the comparisons (58 of 70) favored
active adults, but half the results did not reach statistical significance,
often because of too small sample sizes. The average number of people for each
comparison was approximately 1,300 people, but a fourth of the comparisons had
700 people or less. Figure G8.5 illustrates 18 crude and/or adjusted odds
ratios and 95% confidence intervals from the 13 prospective cohort studies of
physical activity and distress or well-being in more than 100,000 adults from 8
countries, including 67,000 American women (23;94).
Does Physical Activity Reduce Feelings of
Distress or Enhance Feelings of Well-Being?
Conclusions
The effects of RCTs indicate small benefits of physical activity that
often do not exceed the effects of placebo control conditions, such as health
education or stretching.
Rationale
Since 1995, at least 26 RCTs, including nearly 3,000 people, have
examined the effects of exercise on feelings of distress or well-being in
healthy adults or adults with medical conditions other than psychiatric
disorders or disabling conditions that severely limit physical activity (i.e.,
spinal cord injury, multiple sclerosis, and stroke or severe head trauma). The
average effect of exercise compared to a control condition was 0.27 SD
(95% CI = 0.16 to 0.38). The outcomes were favorable after
exercise in nearly 80 percent of the comparisons (26 of 33) with control
conditions, but only 13 of 33 comparisons reached statistical significance. The
average sample size was about 60 people, but a fourth of the studies had less
than 45, a much smaller number than the 200 or so people needed to detect an
effect as small as one-third SD. When compared to a placebo (usually stretching
or health education), the effect of exercise was reduced to 0.10 SD (95% CI:
−0.12 to 0.32) and was significant in just 2 of 9 comparisons.
Figure G8.5 Feelings of Distress/Well-Being:
Prospective Cohort Studies, 1995 Through 2007: Crude and Adjusted Odds
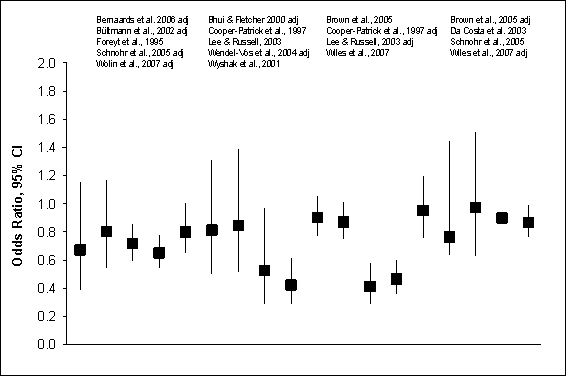
Figure G8.5 Data Points
| Author |
Year |
High |
Low |
Odds Ratio |
| Bernaards et al. |
2006 adj |
1.155 |
0.391 |
0.6719 |
| Bhui & Fletcher |
2000 adj |
1.169 |
0.548 |
0.8008 |
| Brown et al. |
2005 |
0.858 |
0.597 |
0.716 |
| Brown et al. |
2005 adj |
0.775 |
0.545 |
0.65 |
| Bültmann et al. |
2002 adj |
1 |
0.65 |
0.8 |
| Cooper-Patrick et al. |
1997 |
1.311 |
0.503 |
0.8118 |
| Cooper-Patrick et al. |
1997 adj |
1.385 |
0.515 |
0.8444 |
| Da Costa et al. |
2003 |
0.968 |
0.287 |
0.527 |
| Foreyt et al. |
1995 |
0.611 |
0.292 |
0.4221 |
| Lee & Russell |
2003 |
1.05 |
0.774 |
0.9015 |
| Lee & Russell |
2003 adj |
1.014 |
0.747 |
0.8699 |
| Schnohr et al. |
2005 |
0.58 |
0.289 |
0.4099 |
| Schnohr et al. |
2005 adj |
0.601 |
0.359 |
0.4648 |
| Wendel-Vos et al. |
2004 adj |
1.201 |
0.755 |
0.9521 |
| Wiles et al. |
2007 |
1.444 |
0.638 |
0.764 |
| Wiles et al. |
2007 adj |
1.507 |
0.63 |
0.9741 |
| Wolin et al. |
2007 adj |
0.935 |
0.863 |
0.8985 |
| Wyshak et al. |
2001 |
0.986 |
0.764 |
0.868 |
Do the Effects of Physical Activity on
Distress or Well-Being Differ According to Race/Ethnicity, Sex, Age, or Medical
Condition?
Conclusions
The available evidence supports that regular physical activity is
associated with reduced feelings of distress and enhanced feelings of
well-being regardless of age, sex, race/ethnicity, or medical condition.
However, whether these factors modify those associations has not been studied.
Race and ethnicity have been poorly represented or not described in most
studies.
Rationale
Cross-sectional samples of Americans in the National Health Interview
Survey (92), the Behavioral Risk Factor Surveillance
Survey (93;95), and the National
Physical Activity and Weight Loss Survey (91) included
African American, Hispanics, and small numbers of Asian Americans and American
Indians, but results were not compared between groups. Most of the prospective
cohort studies were conducted in Europe or Australia and did not describe
minority representation of the sample. The US Nurses’ Health Study
includes minority women, but their representation was not described in the
study of physical activity and well-being in the cohort (94).
Similarly, it is unclear whether sex and age modify the protective
effect of physical activity on distress/well-being. Only 4 cohort studies
reported findings separately for males and females, with mixed results (96-99),
and none compared age groups. Across cohort studies, findings were similar for
men and women. However, age was inversely related to reduced odds of distress
or increased odds of enhanced well-being regardless of sex. After adjustment
for other risk factors, odds were 0.65 (95% CI = 0.61 to 0.72) for
men and women younger than age 55 years and 0.90 (95% CI = 0.86 to
0.97) for men and women aged 55 years and older. In RCTs, the average effect of
exercise compared to a control condition is similar in both healthy adults
(0.28 SD, 95% CI = 0.16 to 0.41) and people with non-psychiatric
medical conditions (0.26, 95% CI: 0.10 to 0.41). Figure G8.6 illustrates effect
sizes and 95% confidence intervals feelings of distress or well-being in
studies comparing exercise with a control condition from the RCTs of adults
with or without medical conditions.
These trials reported similar effects of exercise in both men and women
and in groups varying in mean aged 31 to 74 years. However, the studies were
not designed to compare groups according sex, race/ethnicity, or age. Only 1 of
the trials compared men and women (45), and results were
mixed across the 3 studies of men (0.49 SD, 95% CI = −0.11 to
1.08). Ten studies reported results for women (0.21 SD, 95% CI = 0.02
to 0.40), and 15 studies combined results for similar proportions of men
and women (0.26 SD, 95% CI = 0.12 to 0.41). None compared
effects between people of different ages or race/ethnicities. Seven of 16
studies of Americans described the racial/ethnic composition of their
samples, but only 2 studies included a substantial portion of African Americans
(46;100). Three studies included
small numbers of Hispanics and/or Asian Americans (51;78;100). The small number of studies
precluded meaningful comparisons among the medical conditions.
Figure G8.6 Feelings of Distress/Well-Being:
Randomized Controlled Trials 1995 Through 2007
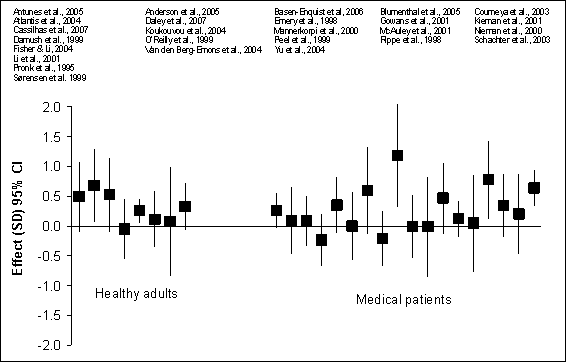
Figure G8.6 Data Points
| Healthy Adults |
Year |
High |
Low |
Effect Size |
| Antunes et al. |
2005 |
1.077 |
−0.095 |
0.491 |
| Atlantis et al. |
2004 |
1.293 |
0.073 |
0.683 |
| Cassilhas et al. |
2007 |
1.144 |
−0.084 |
0.53 |
| Damush et al. |
1999 |
0.453 |
−0.547 |
−0.047 |
| Fisher & Li |
2004 |
0.455 |
0.059 |
0.257 |
| Li et al. |
2001 |
0.58 |
−0.353 |
0.114 |
| Pronk et al. |
1995 |
0.987 |
−0.835 |
0.0758 |
| Sørensen et al |
1999 |
0.721 |
−0.059 |
0.331 |
| Medical Patients |
Year |
High |
Low |
Effect Size |
| Anderson et al. |
2005 |
0.549 |
−0.019 |
0.265 |
| Basen-Enquist et al. |
2006 |
0.641 |
−0.465 |
0.088 |
| Blumenthal et al. |
2005 |
0.496 |
−0.332 |
0.082 |
| Courneya et al., |
2003 |
0.2 |
−0.666 |
−0.233 |
| Daley et al. |
2007 |
0.817 |
−0.115 |
0.351 |
| Emery et al. |
1998 |
0.566 |
−0.566 |
0 |
| Gowans et al. |
2001 |
1.319 |
−0.119 |
0.6 |
| Kiernan et al. |
2001 |
0.246 |
−0.666 |
−0.21 |
| Koukouvou et al. |
2004 |
2.037 |
0.331 |
1.1837 |
| Mannerkorpi et al. |
2000 |
0.511 |
−0.527 |
−0.008 |
| McAuley et al. |
2001 |
0.825 |
−0.85 |
−0.0123 |
| Nieman et al. |
2000 |
1.06 |
−0.121 |
0.4695 |
| O’Reilly et al. |
1999 |
0.42 |
−0.176 |
0.122 |
| Peel et al. |
1999 |
0.854 |
−0.754 |
0.05 |
| Rippe et al. |
1998 |
1.43 |
0.12 |
0.775 |
| Schachter et al. |
2003 |
0.866 |
−0.17 |
0.348 |
| Van den Berg-Emons et al. |
2004 |
0.88 |
−0.468 |
0.206 |
| Yu et al. |
2004 |
0.934 |
0.344 |
0.639 |
Do the Effects of Physical Activity Vary
According to Features of Physical Activity Including Type, Intensity, or Timing
(i.e., Session Duration, Weekly Frequency, and Length of Participation)?
Conclusions
Population-based studies indicate that participation in either moderate
or high levels of physical activity is associated with reduced feelings of
distress or enhanced well-being, when compared with inactivity or very low
physical activity exposure. The minimal or optimal type or amount of exercise
for reducing feelings of distress or enhancing feelings of well-being are not
yet known, but it appears that an increase in physical fitness is not
required.
Rationale
Seven prospective cohort studies of feelings of distress or well-being
included 3 or more levels of physical activity, using inactivity or low
activity as the reference group (18;55;56;94;96;97;101).
Independently of age and sex, and with or without adjustment for other risk
factors, a linear reduction in odds of about 10% occurred for each level of
physical activity compared to people who were inactive or had very low
activity. Similarly, odds favored people who met or exceeded recommendations
for moderate or vigorous participation by about 5% compared to active people
who did not meet either recommended level. No studies permitted a direct
comparison of moderate and vigorous recommended levels, but unadjusted odds
were lower for participation in moderate-to-vigorous physical activity
(OR = 0.77, 95% CI = 0.70 to 0.84) compared to
participation at levels less than recommended (OR = 0.84, 95%
CI = 0.78 to 0.91). Figure G8.7 shows the odds ratios and 95% CI in
prospective cohort studies that examined the dose-response association between
levels of physical activity and feelings of distress or well-being.
A few RCTs manipulated type (walking versus aquatic exercise or Qigong)
or timing (i.e., intermittent versus continuous) of exercise to examine whether
those feature modify the effects of exercise on feelings of distress or
well-being (60-62;64), but the
studies did not include a control group who did not exercise. About 45 percent
of the controlled studies used an aerobic exercise intervention such as
walking, jogging, cycling, or aquatic exercise, and another 30 percent of the
studies combined aerobic activity with resistance exercise. Only 3 studies used
resistance exercise alone, and none compared aerobic with resistance exercise.
About half the studies used continuous exercise, and a third used intermittent
exercise. One study of home-based aerobic dance reported positive effects when
daily exercise was a continuous 30-minute session but not when it was 2
sessions of 15 minutes each separated by 4 hours (80).
Timing of the exercise could not be determined in the other studies. Effects of
exercise did not differ according to the mode or timing of exercise.
Figure G8.7 Feelings of Distress/Well-Being:
Prospective Cohort Studies 1995 Through 2007: Dose Response
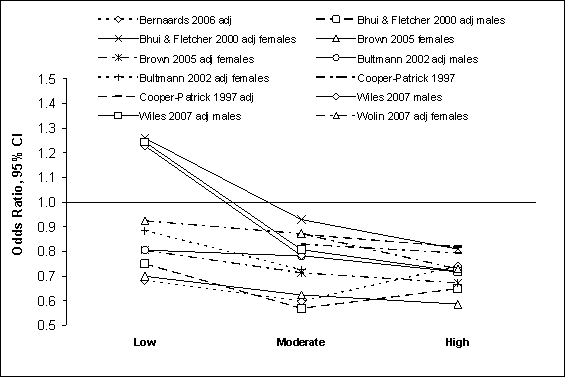
Figure G8.7 Data Points
| Author/Year |
Low |
Moderate |
High |
| Bernaards 2006 adj |
0.6829 |
0.599 |
0.7416 |
| Bhui & Fletcher 2000 adj males |
0.75 |
0.57 |
0.65 |
| Bhui & Fletcher 2000 adj females |
1.26 |
0.93 |
0.81 |
| Brown 2005 females |
0.7 |
0.624 |
0.587 |
| Brown 2005 adj females |
0.805 |
0.714 |
0.673 |
| Bultmann 2002 adj males |
0.806 |
0.782 |
|
| Bultmann 2002 adj females |
0.885 |
0.726 |
|
| Cooper-Patrick 1997 |
|
0.83 |
0.794 |
| Cooper-Patrick 1997 adj |
|
0.87 |
0.8196 |
| Wiles 2007 males |
1.2288 |
0.7833 |
0.7178 |
| Wiles 2007 adj males |
1.244 |
0.8079 |
0.72 |
| Wolin 2007 adj females |
0.9247 |
0.8729 |
0.7298 |
Three-fourths of the studies used a moderate-to-vigorous exercise
intensity of 60% to 80% of people’s aerobic capacity or maximum strength.
The average session lasted 45 minutes and occurred 3 days per week, but studies
had a wide range of session duration (10 to 85 minutes) and weekly frequency (1
to 7 days). Nonetheless, these features of exercise did not modify effects
independently of each other. Studies lasted an average of 6 months, and a
fourth of the studies lasted less than 3 months. Length of the exercise program
was also unrelated to symptom outcome.
Eleven of 13 studies that measured cardiorespiratory fitness reported a
significant increase that averaged 1 SD, or about 20%. Strength was
significantly increased in each of 3 studies that measured strength. However,
there was no association between fitness increases and changes in feelings of
distress or well-being.
Question 4: Is There an Association Between
Physical Activity and Cognitive Function and Dementia?
Introduction
Cognition can be conceptualized as processes involved in selecting,
manipulating, and storing information derived from experiences and how these
processes guide behavior. Cognitive abilities are functional properties of the
individual that are not directly observed but are inferred from behavior.
Researchers in the disciplines of psychometrics, cognitive psychology, and
neuropsychology have developed more than 400 tests designed to assess specific
types of mental processing (102). They range from those
designed to evaluate specific processes (e.g., working memory,
information-processing speed, inhibition) to those that assess global mental
functioning involving multiple processes. Assessment methods include those
designed specifically to evaluate the effects of injury and degenerative
disease on cognitive function and those designed to evaluate individual
differences in healthy individuals. The multidimensionality of cognitive
function and the diversity of assessment methods present a special challenge
for the interpretation of the evidence about the effect of physical activity
and exercise on cognitive function.
Does Physical Activity Protect Against the
Onset of Age-Related Decline in Cognitive Function or Dementia?
Conclusions
The weight of the available evidence from prospective cohort studies
supports the conclusion that physical activity delays the incidence of dementia
and the onset cognitive decline associated with aging.
Rationale: Prospective Cohort Studies
At least 17 prospective population-based cohort studies have been
published since 1995 that have assessed the association of individuals’
level of physical activity with the onset of age-related decline in cognitive
functioning among healthy adults or with incident cases of dementia. Studies
that confounded the measure of physical activity with other leisure or mental
activities were not considered. Four studies showed protective effects against
cognitive decline in healthy aging adults (103-106) and
two studies did not find protective effects (107;108).
Of the 11 studies of dementia, 7 reported a protective effect of
physical activity. Nine of 16 comparisons were statistically significant (mean
OR = 0.63 95% CI = 0.50 to 0.80). Though limited in number,
results were stronger for Alzheimer’s disease than for other dementias,
including vascular dementia. Figure G8.8 describes results from prospective
cohort studies of physical activity and incident dementia or Alzheimer’s
disease.
The studies varied considerably in sample size, methods used to assess
participant’s physical activity level and mental function, and the
duration between baseline and follow-up measurements. In general, most studies
with sample sizes greater than 1,000 individuals report that physical activity
delays the onset of cognitive decline or dementia. The results of studies
conducted with smaller numbers of participants are inconsistent. The lack of
agreement among the studies reviewed may be explained, at least in part, in
terms of statistical power. An alternative explanation for the inconsistencies
among the results of these studies is the confounding influence of cognitive
stimulation derived while engaged in physical activities (108). In studies that report a relation between physical
activity and delayed onset of dementia, the effects were detected using both
clinical assessments and standardized measures of cognitive function. Further,
early-life (103), mid-life (109),
and current levels of physical activity all appear to postpone symptoms of
dementia. Thus, although exercise does not prevent dementia, it may be
associated with a delay in its onset, perhaps by maintaining a higher level of
cognitive function for physically active adults than less physically active
individuals as they age (110).
Rationale: Randomized Controlled Trials
A meta-analysis of 18 RCTs (111) indicated that
aerobic exercise training produced an effect size (SD) of 0.48 for improving
performance on all cognitive tasks, with the greatest effect size (0.68) for
executive processing tasks that measure goal-oriented decision-making behavior,
compared to 0.46 for controlled-processing tasks that assess attentional
effort, 0.42 for visuospatial tasks that evaluate the perceptual organization,
and 0.27 for speeded tasks that focus on rapid responses and movements. The
results of 2 recently conducted experiments provide support for the
facilitative effects of resistance exercise training on information-processing
functions of cognitively healthy older adults (85;112), though one experiment found no significant effects of
either yoga training or a walking program on older adults’ memory or
information-processing speed (113).
Figure G8.8 Incident Total Dementia or
Alzheimer’s Disease: Prospective Cohort Studies, 1995 Through 2007: Crude
and Adjusted Hazard
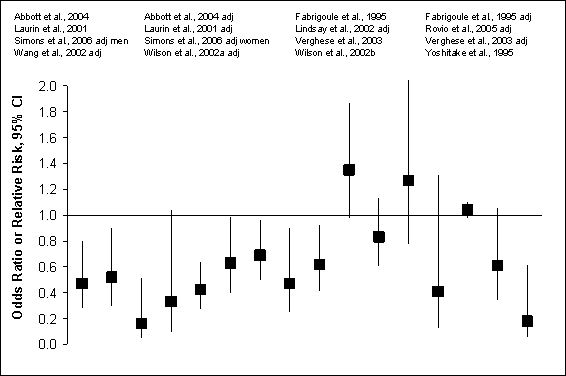
Figure G8.8 Data Points
| Author/Date |
High |
Low |
Odds Ratio or Relative
Risk |
| Abbott et al. 2004 |
0.8 |
0.28 |
0.47 |
| Abbott et al. 2004 adj |
0.9 |
0.3 |
0.52 |
| Fabrigoule et al. 1995 |
0.51 |
0.05 |
0.16 |
| Fabrigoule et al. 1995 adj |
1.04 |
0.1 |
0.33 |
| Laurin et al. 2001 |
0.64 |
0.28 |
0.425 |
| Laurin et al. 2001 adj |
0.98 |
0.4 |
0.63 |
| Lindsay et al. 2002 adj |
0.96 |
0.5 |
0.69 |
| Rovio et al. 2005 adj |
0.9 |
0.25 |
0.47 |
| Simons et al. 2006 adj men |
0.92 |
0.42 |
0.62 |
| Simons et al. 2006 adj women |
1.87 |
0.98 |
1.35 |
| Verghese et al. 2003 |
1.137 |
0.609 |
0.832 |
| Verghese et al. 2003 adj |
2.06 |
0.78 |
1.27 |
| Wang et al. 2002 adj |
1.31 |
0.13 |
0.41 |
| Wilson et al. 2002a adj |
1.1 |
0.98 |
1.04 |
| Wilson et al. 2002b |
1.05 |
0.35 |
0.61 |
| Yoshitake et al. 1995 |
0.61 |
0.06 |
0.18 |
Does Physical Activity Reduce Symptoms
Associated With Alzheimer’s Disease or Other Dementias?
Conclusions
Evidence from RCTs of healthy older adults and people with
Alzheimer’s disease or other dementias support that regular participation
in physical activity improves aspects of cognitive function or reduces symptoms
of dementia.
Rationale
A meta-analytic review of 10 randomized controlled studies of older
adults diagnosed with cognitive impairments (114)
concluded that physical activity interventions benefit the cognitive function
of older adults regardless of disease status. Similar to the finding of
Colcombe and Kramer (111) with healthy older adults,
older adults with impairments who participated in physical activity
interventions show better cognitive function than those assigned to control
conditions (ES = .57). However, concerns of the methodological
integrity of many of the studies reviewed led the authors to recommend caution
in the interpretation of these findings. The beneficial effects of physical
activity on older adults with dementia have been reported in 2 recent
experiments (115;116).
Although individuals with dementia benefit cognitively from
participation in exercise training, it is not clear whether physical activity
ameliorates symptoms of dementia or simply maintains the level of cognitive
function for those who are active. In several experiments, differences in
cognitive function and information-processing performance in older adults
assigned to physical activity or control conditions was due to the degradation
of performance by inactive individuals. Further, although relatively short-term
exercise programs demonstrate improvements on older adults’ cognitive
function, the durability of the effect remains to be determined.
Do the Effects of Physical Activity on
Cognitive Function Differ According to Genetics, Age, Sex, Race/Ethnicity, or
Medical Condition?
Conclusions
The available evidence supports the benefits of physical activity for
improving or maintaining cognitive function among healthy older adults, more so
than for young adults whose cognitive health is normally near peak function.
The smaller, but generally favorable effects of physical activity on cognitive
function and symptoms among people with dementia may be modified by the
severity of dementia and other health factors.
Rationale
The protein apolipoprotein E4 (ApoE4) is a known risk factor for
Alzheimer’s disease. Individuals who have two copies of the ApoE4 allele
are at greatest risk for the disease, followed by individuals with one copy of
the ApoE4 allele, followed by those who are non-carriers. Investigators have
suggested that physical activity diminishes the effects of ApoE4 on the
development of Alzheimer’s disease. The evidence from prospective cohort
studies has been inconsistent, however, with some reporting that physical
activity benefits the cognitive functioning of ApoE4 non-carriers and has no
effect for ApoE4 carriers (117), and others report that
physical activity benefits cognitive functioning for ApoE4 carriers, but has no
effect for ApoE4 non-carriers (117;118). Nevertheless, evidence that genetic factors may
influence the relation between physical activity and cognition is important as
it may help to explain some of the inconsistencies among studies that have
examined the effects of physical activity on cognitive performance in the
general population of older adults.
The results of one of 15 prospective cohort studies reviewed suggest
that sex may modify the relation between physical activity experiences and
cognitive function (103). Older Dutch men, but not women,
who reported being physical active early in their lives (ages 15 to 25 years)
had faster information-processing abilities than those who were less physically
active during those years of their lives.
The benefits of physical activity on the cognitive function of older
adults who have dementia may be reduced in individuals with cardiovascular risk
factors. Daily walking or gardening reduced the 16-year combined risk of
Alzheimer’s and vascular dementia among elderly male residents of Dubbo,
New South Wales in Australia but only daily gardening was protective among
females (119). An evaluation of the changes in brain
structure and function that accompany Alzheimer’s disease and changes in
cerebral metabolic processes that accompany ischemia led Eggermont and
colleagues (120) to speculate that an individual’s
cardiovascular risk factors may attenuate or even reverse the positive effects
of exercise on cognition and that participation in structured exercise programs
may not be beneficial for all patients. Thus, while the bulk of scientific
evidence obtained thus far provides compelling support for the benefits of
physical activity for healthy older adults, the favorable effects of physical
activity may be modified by cardiovascular health or risk. Additionally, the
gains in cognitive function brought about by physical activity may be observed
more clearly in older adults than young adults, whose level of cognitive health
is near its peak and provides less room for exercise-related improvement (121).
Do the Effects of Physical Activity Vary
According to Features of Physical Activity, Including Type, Intensity, or
Timing (i.e., Session Duration, Weekly Frequency, and Length of
Participation)?
Conclusions
Evidence from prospective cohort studies or RCTs is insufficient to
determine whether cognitive function or symptoms of dementia vary according to
features of physical activity exposure.
Rationale
Four of 6 prospective cohort studies of cognitive decline with aging in
healthy people examined more than 2 levels of physical activity exposure. Three
of them, including a large cohort of women from the Nurses’ Health Study
(105), found a dose-response relation, whereby the
decline in risk was greater at higher levels of physical activity
participation. Four of 10 prospective cohort studies of dementia risk examined
more than 2 levels of physical activity exposure. Two of the 4 studies reported
a dose-response relation such that higher levels of physical activity result in
greater protection against dementia (122;123). The other 2 studies of dementia (124;125) reported similar trends, but
the cohorts were too small to detect statistical significance.
The methods used to measure physical activity vary greatly, both among
RCTs and prospective cohort studies, which makes it difficult to identify the
effects of specific features of physical activity on cognitive function. The
use of self-report measures of physical activity is a limitation of prospective
studies in general, but it is a unique problem for studies of cognitive
function when the measures of physical activity include leisure activities that
involve social and cognitive activities but light physical exertion (e.g.,
crossword puzzles, card games).
Cognitive function has been associated with levels of and gains in
cardiorespiratory fitness among healthy, older adults (126-128), and fitness also has been positively correlated
with white matter integrity (129), brain volume (130;131), and hippocampal neurogenesis
(132). However, the cumulative evidence does not provide
clear support that cardiorespiratory fitness is associated with cognitive
performance (133).
The sole randomized controlled experiment designed specifically to
assess the dose-effect relation between resistance training and information
processing in healthy older adults reported that similar benefits were derived
from interventions that involved weight loads of either 50% or 80% of maximal
strength (85). The results of a large prospective cohort
study, led Podewils and colleagues (117) to conclude
that, with respect to the onset of dementia, the number of different physical
activities in which older adults engage may be more important than their
frequency, intensity, or duration. At this time, the dose-response relation
among mode, duration, and intensity of exercise training and changes in
cognition remains unclear.
Question 5: Is There an Association Between
Physical Activity and Sleep?
Introduction
Nearly one-third of the adult population in the United States
experiences insomnia every year, and each year 50 to 70 million Americans
experience some effects on their health from sleep disorders, sleep
deprivation, and excessive daytime sleepiness (134). The
financial cost is approximately $65 billion, $50 billion of which represents
costs to industry from lost productivity. Approximately 70 sleep disorders
exist, the most studied of which are insomnia and obstructive sleep apnea. Many
neurological disorders are associated with poor sleep, and poor sleep itself
can have important health-related outcomes. Only about 5% to 20% of people who
suffer sleep disturbances will seek help from a primary care physician, and
many will purchase over-the-counter sleep aids.
Does Physical Activity Protect Against the
Onset of Insomnia or Other Sleep Problems?
Conclusions
A small number of observational, population-based studies provides
initial evidence supporting a positive association of regular participation in
physical activity with lower odds of disrupted or insufficient sleep, including
sleep apnea.
Rationale
Population-based studies have found that physically active people report
better sleep than inactive people (135), but most of the
studies used a cross-sectional design and do not permit conclusions about the
temporal sequence of physical activity and sleep. One prospective cohort study
(136) found that those who reported more physical
activity had nearly 40% lower odds of incident insomnia. Eleven of 13
cross-sectional studies show that the chances of having insufficient or
interrupted sleep are lower (mean OR = 0.73 95% CI = 0.66
to 0.81) among adults who are engaged in more physical activity than among
those who have less physical activity or are sedentary (136-146). At least 2 population-based, cross-sectional
studies found that men and women who exercise at least 3 hours per week had
lower odds of sleep apnea measured by polysomnography (147;148). Figure G8.9 shows odds
ratios from population-based cross-sectional studies of physical activity and
self-ratings of disrupted or insufficient sleep.
Does Physical Activity Reduce Sleep
Problems and Enhance Sleep Quality?
Conclusions
The weight of the evidence from a small number of RCTs supports the
conclusion that regular participation in physical activity has favorable
effects on sleep quality and is a useful component of good sleep hygiene.
Rationale
Six RCTs, all published since 1995, show positive and large effects
(more than 1 SD) of exercise training on symptoms of poor sleep and self-rated
sleep quality (26;37;149-152). Acute aerobic exercise of varying intensities
elicits small to moderate improvements (approximately 4 to 13 minutes) in
several features of objectively measured sleep quality, including increases in
time spent in slow-wave sleep, total sleep time, and latency for rapid eye
movement (REM) sleep, and a decrease in REM sleep among normal sleepers (153). The effects for total sleep time are larger when the
exercise lasts more than an hour but do not vary according to people’s
fitness levels or the intensity of the exercise. The effects of a single
exercise session on the sleep of people with sleep disorders are not known. The
long-term effects of exercise on objective measures of sleep among poor
sleepers have not been studied much (154), and the
results of a few RCTs (155-157) and quasi-experimental
studies (158;159) of nursing home
residents or patients with sleep apnea have been mixed.
Figure G8.9 Physical Activity and Symptoms of
Disrupted or Insufficient Sleep: 13 Cross-Sectional Studies (Total
n=84,904)
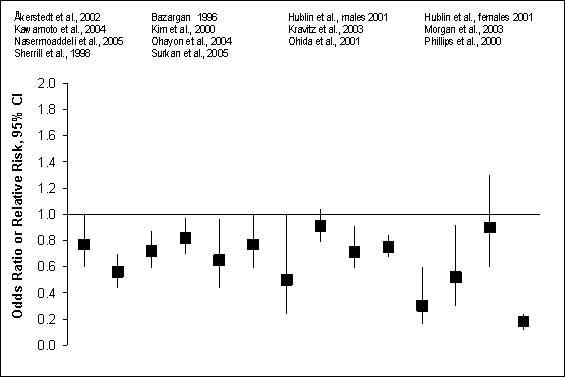
Figure G8.9 Data Points
| Author/Date |
High |
Low |
Odds Ratio or Relative
Risk |
| Åkerstedt et al., 2002 |
1 |
0.6 |
0.77 |
| Bazargan
1996 |
0.697 |
0.44 |
0.56 |
| Hublin et al., males 2001 |
0.87 |
0.59 |
0.72 |
| Hublin et al., females 2001 |
0.97 |
0.7 |
0.82 |
| Kawamoto et al., 2004 |
0.96 |
0.44 |
0.65 |
| Kim et al., 2000 |
1 |
0.59 |
0.77 |
| Kravitz et al., 2003 |
1 |
0.24 |
0.5 |
| Morgan et al., 2003 |
1.04 |
0.79 |
0.91 |
| Nasermoaddeli et al., 2005 |
0.91 |
0.59 |
0.71 |
| Ohayon et al., 2004 |
0.84 |
0.68 |
0.75 |
| Ohida et al., 2001 |
0.6 |
0.16 |
0.3 |
| Phillips et al., 2000 |
0.92 |
0.3 |
0.52 |
| Sherrill et al., 1998 |
1.3 |
0.6 |
0.9 |
| Surkan et al., 2005 |
0.61 |
0.06 |
0.18 |
Do the Effects of Physical Activity on
Sleep Differ According to Age, Sex, Race/Ethnicity, or Medical Condition?
Conclusions
The few RCTs and the scarcity of prospective cohort studies do not
permit conclusions about whether the effects of physical activity on sleep
differ according to types of people, age, type of sleep disorder, or other
medical conditions.
Rationale
Self-reports of exercise frequency and sleep problems were inversely
related in a cross-sectional study of a thousand elderly African American
residents of New Orleans (138). Older, sedentary adults
without cardiovascular disease who complained of sleep problems reported
improvements in self-rated sleep after a 16-week exercise program of moderate
intensity consisting of 30 to 40 minutes of low-impact aerobics and brisk
walking 4 times a week (150). Similarly, older, depressed
adults with sleep problems who were not currently being medically treated
reported improvements in self-rated sleep that was accompanied by a reduction
in depression symptoms (160) after a 10-week,
3-days-per-week, moderately intense (80% of one maximum repetition) resistance
training program (26).
Do the Effects of Physical Activity Vary
According to Features of Physical Activity Including Type, Intensity, or Timing
(i.e., Session Duration, Weekly Frequency, and Length of Participation)?
Conclusions
Evidence from prospective cohort studies or RCTs is insufficient to
determine whether features of sleep quality vary according to features of
physical activity exposure.
Rationale
In a year-long study of moderate intensity (60% to 85% maximal heart
rate) walking or leg cycling exercise (45 minutes or more, 5 or more days each
week) among postmenopausal, overweight or obese, sedentary women not taking
hormone replacement therapy, morning exercisers who exercised at least 225
minutes per week reported less trouble falling asleep compared with those who
exercised less than 180 minutes per week. In contrast, evening exercisers who
exercised at least 225 minutes per week reported more trouble falling asleep
compared to those who exercised less than 180 minutes per week (152). However, those differences might have been biased, as
the timing of exercise sessions were self-selected and most evening exercisers
were employed while the morning exercisers were mostly retired or not working.
Regardless, increases in cardiorespiratory fitness were related to reduced odds
of poor sleep quality, independently of changes in body mass index or time
spent outdoors.
Fifteen, 60-minute sessions once a week that emphasized
moderate-intensity recreational sports activities (e.g., softball, dance,
self-defense, swimming, and athletics) were accompanied by a small increase in
self-rating of total time spent sleeping among older, sedentary adults who were
group-randomized according to age and socioeconomic status (161). Other studies of less vigorous exercises, such as
walking or yoga, have reported smaller or statistically non-significant
reductions in self-rated sleep (47;162;163).
Question 6: Is There an Association Between
Physical Activity and Other Aspects of Mental Health?
Introduction
Physical activity and exercise may have the potential to reduce the
onset or progression of central nervous system disorders other than dementia
that contribute to disability and mortality risk, such as multiple sclerosis
(164) and Parkinson’s disease (165). They also may reduce the adverse impact of these
disorders on quality of life. However, too few prospective cohort studies and
RCTs have been conducted to allow conclusions about the protective effects of
physical activity for CNS diseases other than Alzheimer’s disease and
other dementias.
Benefits of physical activity may also extend to other aspects of mental
health that are less directly linked to disability and mortality risks but are
important contributors to overall quality of life, such as self-esteem and
feelings of energy/fatigue. Sufficient evidence exists to encourage more study
in these areas, but presently not enough studies are available to draw
conclusions about how the effects of physical activity or exercise might differ
according to types of people or types and amounts of physical activity.
Self-Esteem
Enhanced self-esteem has significance for mental health because it
conveys a feeling of value or self-worth and it is a generalized indicator of
psychological adjustment and health risk (89). A
meta-analysis of about 50, mostly small, RCTs of various types of exercise
reported an average increase in self-esteem of about 0.25 SD among adults (166). Self-esteem is increased among adults (166) when physical fitness is increased. However, because
features of physical activity exposure were not associated with self-esteem
outcomes in the studies, it is difficult to conclude that fitness influences
self-esteem independently of other diverse aspects of the studies’
methods, social contexts, and participant expectations of benefit. Nonetheless,
larger gains in self-esteem can be expected for individuals with low initial
levels, and for whom physical attributes have a relatively high value as a part
of global self-concept.
Chronic Fatigue
The literature on physical activity and chronic fatigue syndrome is
small, and epidemiologic studies of this condition rarely have included
measures of physical activity. Five relatively small RCTs all show a positive
effect of exercise training on symptoms of chronic fatigue syndrome (167-171). A dozen population-based observational studies,
including 4 prospective cohorts published since 1995, suggest a protective
effect of physical activity against feelings of fatigue or low energy
(OR = 0.61, 95% CI = 0.52 to 0.72) (172), and RCTs of groups of medical patients and other
adults show a moderate reduction in symptoms of fatigue (173;174).
Question 7: Is There an Association Between
Physical Activity and Adverse Psychological Events?
Some adverse psychological events have been reported among extremely
active people, but whether they are causally influenced by physical activity
exposure is not yet known. Clinical cases of “running addiction,”
were described 30 years ago (175), whereby motivation for
running exceeded commitments to work, family, social relations, and medical
advice. Similar cases have been labeled positive addiction, runner’s
gluttony, fitness fanaticism, athlete’s neurosis, obligatory running,
and exercise abuse.
However, little is understood about the origins, valid diagnosis, or
mental health impact of exercise abuse (176;177). Though exercise abuse or addiction is a recognized
problem for clinical medicine, its population prevalence is unknown but likely
low. Likewise, it is not known whether excessive exercise and disordered eating
share a common course that is motivated by common goals and followed by common
medical outcomes. It has been proposed that anorexia athletica is a subclinical
syndrome of anorexia nervosa (178), but the prevalence of
disordered eating and the independent risk caused by sport and exercise have
not yet been established by controlled epidemiologic and clinical studies. In
most cases, the eating behaviors of athletes do not appear to signal anorexia
nervosa or bulimia (i.e., bingeing and purging (179)),
which have prevalence rates in the United States of about 1% and 4%,
respectively. Cases of muscle dysmorphia, a proposed form of body dysmorphic
disorder, in which a person develops a pathological preoccupation with his or
her muscularity and has associated symptoms of severe subjective distress,
impaired social and occupational functioning, and abuse of anabolic steroids
and other substances, have been reported (180;181). No population-based studies have been conducted to
determine the prevalence of muscle dysmorphia nor any prospective cohort
studies or randomized clinical studies to determine whether muscle dysmorphia
or body dysmorphia results from participation in resistance exercise training
or whether people who have existing vulnerability to a distorted body image and
self-concept are drawn to weightlifting.
Results from nearly 100 studies on these potential adverse events of
participation in exercise training and sports are inconclusive because the
studies described symptoms among different groups of active or inactive people
without fully considering attributes other than activity history that might
account for abusive exercise or eating problems (177).
Risk profiles of exercise abusers or athletes have not been evaluated against
those of non-athletes from the same academic, socioeconomic, or psychological
backgrounds. The studies often lack standard definitions or valid measures of
physical activity or disordered eating. Though anorexics often augment food
restriction by hyperactivity, their aerobic fitness is well below average
because of muscle wasting and anemia, in contrast to the above-average aerobic
fitness of habitual runners and trained athletes. In addition, cross-sectional
studies have not revealed a common psychopathology between obligatory (i.e.,
excessively committed) runners and anorexic patients.
Anxiety can be elevated slightly immediately after maximal exercise
testing or heavy resistance exercise (i.e., approximately 80% of 1 repetition
maximum (182;183)), but that
elevation is temporary. Nonetheless, overall reductions in anxiety can be
delayed by 1 or 2 hours after resistance exercise is completed. Evidence from
15 studies refutes the potential association between exercise and panic
attacks; only 5 panic attacks were reported during exercise involving 444
exercise bouts performed by 420 panic disorder patients (184). Research has also shown that lactate accumulation
resulting from exercise is not related to increased risk of panic attacks among
patients with panic disorder (185) or postexercise
anxiety in normal individuals (186). Moreover, a small,
randomized clinical trial recently showed that 10 weeks of aerobic exercise
training was effective in reducing symptoms of anxiety among patients with
panic disorder and agoraphobia (72).
Question 8: What Mechanisms Can Plausibly
Explain the Association Between Physical Activity and Mental Health?
Very limited evidence exists about biological mechanisms that can
explain the effects of physical activity or exercise on mental health (187). It is accepted that the brain and the rest of the
central nervous system regulate moods, emotions, cognitions, sleep, and
neurological functions, and that social and environmental factors interact with
genes to regulate the brain. Experimental animal studies show that both
voluntary and forced running activate and produce adaptations in several
aspects of brain neural circuits involved with learning, memory, motivation,
and behaviors that mimic features of human depression, anxiety, and cognitive
function. These include neurotransmitters (e.g., acetylcholine, glutamate, and
gamma-aminobutyric acid), neuromodulators (e.g., dopamine, norepinephrine, and
serotonin) and their receptors, neuropeptides that influence neurotransmission
(e.g., galanin and neuropeptide Y), and neuronal growth factors (e.g., brain
derived neurotrophic factor and VGF), all of which are targets of experimental
treatments for depression and anxiety. However, only a few studies have used
animal models of mental or neurological disorders, testing whether brain and
behavior changes coincide as predicted after physical activity (e.g.,
188-194). Likewise, the way in which exercise regulates
sleep has received little study and is unknown. Indirect evidence suggests that
acute bouts of exercise are positively associated with melatonin production in
women (195) and can induce circadian phase shifts (196) including a phase delay in circadian melatonin rhythm
(197), influence adenosine metabolism (198), and activate brain neurological circuits hypothesized
to help people feel less anxious and depressed (154).
Unlike other chronic diseases, non-biological mechanisms also may
explain the effect of physical activity on mental health. Cognitive
explanations include increased self-esteem (e.g., 199),
perceptions of social support, and efficacy beliefs about personal control.
Similarly, acute responses to single exposures of physical activity, such as
transient changes in moods (200;201) or feelings of energy; pleasant (e.g., enjoyment) or
unpleasant (e.g., discomfort or pain) affective experiences during exercise; or
nightly sleep (153), are likely to contribute to the
accumulative psychological responses that typically are measured only after
chronic exposure to physical activity. Also such responses might contribute
indirectly by increasing exposure to physical activity (e.g. better adherence
to exercise) or by influencing other factors important to mental health (e.g.,
social engagement). Tests of those contributions will require research designs
and methodologies that are difficult to implement with tight control in
clinical or public health settings (e.g., 202-206). Thus,
continued experimental research on biological mechanisms and innovative tests
of social or cognitive mediators of psychological responses are needed to
confirm and explain the evidence from prospective observational studies and
RCTs that physical activity and exercise promote mental health.
Overall Summary and Conclusions
The weight of the cumulative evidence from prospective cohort studies
and RCTs of uneven quality supports the conclusion that physical activity has
protective benefits for several aspects of mental health. The evidence is
strongest for protection against symptoms of depression and cognitive decline
associated with aging, including the onset of dementia. Substantial evidence
also suggests that physical activity reduces symptoms of anxiety and poor
sleep, as well as feelings of distress and fatigue, and enhances well-being.
Physical activity generally appears equally beneficial for adults regardless of
age, sex, race/ethnicity, or health status, but few studies have directly
compared benefits or hazards of physical activity among those population
segments. Minority groups have been poorly represented in most studies. A small
number of prospective cohort studies suggest that health benefits for
depression and dementia are greater with higher levels of physical activity
exposure, but insufficient evidence precludes conclusions about the minimal or
optimal types or amounts of physical activity for mental health. Benefits do
not appear to depend on fitness or fitness gains. Current evidence supports the
conclusion that regular participation in moderate-to-vigorous physical
activity, consistent with current public health guidelines, confers mental
health benefits when compared to participation in low levels of physical
activity or a sedentary lifestyle.
Research Needs
This review of physical activity and mental health identified a number
of research needs. First, more prospective cohort studies and tightly
controlled RCTs are needed, especially for anxiety and sleep disorders.
Specifically:
- More studies of under-represented groups and of people at high risk
of mental health disorders are needed.
- Selection of potential confounders specific to mental health risks
need to be included in prospective cohort studies.
- Reporting of adherence to and dropout from trials should be improved,
particularly with respect to the impact on the trial’s efficacy and likely
population effectiveness.
- Investigators should strive for convergence of subjective and
objective measures of physical activity and should specify the social and
environmental contexts in which physical activity occurs.
- Valid outcome measures need to be selected, refined, and used
uniformly.
- Physical activity exposures and outcomes need to be measured
frequently to permit investigators to model change.
- It would be helpful to conduct additional RCTs comparing the effects
of exercise with other preventive interventions.
- Novel designs that distinguish social moderators and mediators of
outcomes from experimental contamination (i.e., placebo effects) would make a
valuable contribution to the field.
A second important research need is studies that manipulate or directly
compare standardized features of physical activity, including type, intensity,
and timing, with the settings in which activity takes place (e.g., group vs.
solitary, community vs. home, indoor vs. outdoor).
Finally, it would be helpful to accelerate the synergy between human
brain imaging studies and neuroscience studies that use animal models of human
disease. This improved synergy could help elucidate biological mechanisms
underlying the benefits of physical activity to mental health. An increased
emphasis on modeling of social-cognitive mediators of mental health outcomes
and studies of gene-environment interactions also would be valuable additions
to the field.
Reference List
- Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso
J, Angermeyer MC, Benjet C, Bromet E, de GG, de GR, et al. Depression-anxiety
relationships with chronic physical conditions: results from the World Mental
Health Surveys. J.Affect.Disord. 2007 Nov;103(1-3):113-20.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray
CJ. Global and regional burden of disease and risk factors, 2001: systematic
analysis of population health data. Lancet 2006 May 27;367(9524):1747-57.
- Mathers CD, Loncar D. Projections of global
mortality and burden of disease from 2002 to 2030. PLoS.Med. 2006
Nov;3(11):e442.
- Michaud CM, McKenna MT, Begg S, Tomijima N,
Majmudar M, Bulzacchelli MT, Ebrahim S, Ezzati M, Salomon JA, Kreiser JG, et
al. The burden of disease and injury in the United States 1996. Popul.Health
Metr. 2006;4:11.
- Lipsey MW, Wilson DB. Practical meta-analysis.
Thousand Oaks, Calif.: Sage Publications; 2001.
- American Psychiatric Association., American
Psychiatric Association.Task Force on DSM-IV. Diagnostic and statistical manual
of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric
Association; 2000.
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA,
Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the
United States: how did it change between 1990 and 2000? J.Clin.Psychiatry 2003
Dec;64(12):1465-75.
- Kessler RC, Berglund P, Demler O, Jin R,
Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions
of DSM-IV disorders in the National Comorbidity Survey Replication.
Arch.Gen.Psychiatry 2005 Jun;62(6):593-602.
- Egede LE, Nietert PJ, Zheng D. Depression and
all-cause and coronary heart disease mortality among adults with and without
diabetes. Diabetes Care 2005 Jun;28(6):1339-45.
- Goodwin RD. Association between physical activity
and mental disorders among adults in the United States. Prev.Med. 2003
Jun;36(6):698-703.
- Morrato EH, Hill JO, Wyatt HR, Ghushchyan V,
Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for
developing diabetes, 2003. Diabetes Care 2007 Feb;30(2):203-9.
- Portillo CJ, White MC, Baisden K, Dawson C.
Angina, functional impairment and physical inactivity among Mexican-American
women with depressive symptoms. Prog.Cardiovasc.Nurs. 1995;10(3):18-25.
- Taylor MK, Pietrobon R, Pan D, Huff M, Higgins
LD. Healthy people 2010 physical activity guidelines and psychological
symptoms: Evidence from a large nationwide database. J.Phys.Act.Health
2004 Apr;1(2):114-30.
- Wassertheil-Smoller S, Shumaker S, Ockene J,
Talavera GA, Greenland P, Cochrane B, Robbins J, Aragaki A, Dunbar-Jacob J.
Depression and cardiovascular sequelae in postmenopausal women. The Women's
Health Initiative (WHI). Arch.Intern.Med. 2004 Feb 9;164(3):289-98.
- Wise LA, ms-Campbell LL, Palmer JR, Rosenberg L.
Leisure time physical activity in relation to depressive symptoms in the Black
Women's Health Study. Ann.Behav.Med. 2006 Aug;32(1):68-76.
- Beard JR, Heathcote K, Brooks R, Earnest A, Kelly
B. Predictors of mental disorders and their outcome in a community based
cohort. Soc.Psychiatry Psychiatr.Epidemiol. 2007 Aug;42(8):623-30.
- Chen J, Millar WJ. Health effects of physical
activity. Health Rep. 1999;11(1):21-30.
- Cooper-Patrick L, Ford DE, Mead LA, Chang PP,
Klag MJ. Exercise and depression in midlife: a prospective study. Am.J.Public
Health 1997 Apr;87(4):670-3.
- Ford DE, Mead LA, Chang PP, Cooper-Patrick L,
Wang NY, Klag MJ. Depression is a risk factor for coronary artery disease in
men: the precursors study. Arch.Intern.Med. 1998 Jul 13;158(13):1422-6.
- Harris AH, Cronkite R, Moos R. Physical activity,
exercise coping, and depression in a 10-year cohort study of depressed
patients. J.Affect.Disord. 2006 Jul;93(1-3):79-85.
- Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA.
Physical activity reduces the risk of subsequent depression for older adults.
Am.J.Epidemiol. 2002 Aug 15;156(4):328-34.
- Strohle A, Hofler M, Pfister H, Muller AG, Hoyer
J, Wittchen HU, Lieb R. Physical activity and prevalence and incidence of
mental disorders in adolescents and young adults. Psychol.Med. 2007
Nov;37(11):1657-66.
- Wyshak G. Women's college physical activity and
self-reports of physician-diagnosed depression and of current symptoms of
psychiatric distress. J.Womens Health Gend.Based.Med. 2001
May;10(4):363-70.
- Lawlor DA, Hopker SW. The effectiveness of
exercise as an intervention in the management of depression: systematic review
and meta-regression analysis of randomised controlled trials. BMJ 2001 Mar
31;322(7289):763-7.
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE,
Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, et al.
Effects of exercise training on older patients with major depression.
Arch.Intern.Med. 1999 Oct 25;159(19):2349-56.
- Singh NA, Clements KM, Fiatarone MA. A randomized
controlled trial of progressive resistance training in depressed elders.
J.Gerontol.A Biol.Sci.Med.Sci. 1997 Jan;52(1):M27-M35.
- Armstrong K, Edwards H. The effectiveness of a
pram-walking exercise programme in reducing depressive symptomatology for
postnatal women. Int.J.Nurs.Pract. 2004 Aug;10(4):177-94.
- Sims J, Hill K, Davidson S, Gunn J, Huang N.
Exploring the feasibility of a community-based strength training program for
older people with depressive symptoms and its impact on depressive symptoms.
BMC.Geriatr. 2006;6:18.
- Tsang HW, Fung KM, Chan AS, Lee G, Chan F. Effect
of a qigong exercise programme on elderly with depression.
Int.J.Geriatr.Psychiatry 2006 Sep;21(9):890-7.
- Brenes GA, Williamson JD, Messier SP, Rejeski WJ,
Pahor M, Ip E, Penninx BW. Treatment of minor depression in older adults: a
pilot study comparing sertraline and exercise. Aging Ment.Health 2007
Jan;11(1):61-8.
- Chou KL, Lee PW, Yu EC, Macfarlane D, Cheng YH,
Chan SS, Chi I. Effect of Tai Chi on depressive symptoms amongst Chinese older
patients with depressive disorders: a randomized clinical trial.
Int.J.Geriatr.Psychiatry 2004 Nov;19(11):1105-7.
- Dunn AL, Trivedi MH, Kampert JB, Clark CG,
Chambliss HO. Exercise treatment for depression: efficacy and dose response.
Am.J.Prev.Med. 2005 Jan;28(1):1-8.
- Knubben K, Reischies FM, Adli M, Schlattmann P,
Bauer M, Dimeo F. A randomised, controlled study on the effects of a short-term
endurance training programme in patients with major depression. Br.J.Sports
Med. 2007 Jan;41(1):29-33.
- Mather AS, Rodriguez C, Guthrie MF, McHarg AM,
Reid IC, McMurdo ME. Effects of exercise on depressive symptoms in older adults
with poorly responsive depressive disorder: randomised controlled trial.
Br.J.Psychiatry 2002 May;180:411-5.
- Pinchasov BB, Shurgaja AM, Grischin OV, Putilov
AA. Mood and energy regulation in seasonal and non-seasonal depression before
and after midday treatment with physical exercise or bright light. Psychiatry
Res. 2000 Apr 24;94(1):29-42.
- Singh NA, Clements KM, Singh MA. The efficacy of
exercise as a long-term antidepressant in elderly subjects: a randomized,
controlled trial. J.Gerontol.A Biol.Sci.Med.Sci. 2001 Aug;56(8):M497-M504.
- Singh NA, Stavrinos TM, Scarbek Y, Galambos G,
Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low
intensity weight training versus general practitioner care for clinical
depression in older adults. J.Gerontol.A Biol.Sci.Med.Sci. 2005
Jun;60(6):768-76.
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins
L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, et al.
Exercise and pharmacotherapy in the treatment of major depressive disorder.
Psychosom.Med. 2007 Sep;69(7):587-96.
- Babyak M, Blumenthal JA, Herman S, Khatri P,
Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise
treatment for major depression: maintenance of therapeutic benefit at 10
months. Psychosom.Med. 2000 Sep;62(5):633-8.
- Kritz-Silverstein D, Barrett-Connor E, Corbeau C.
Cross-sectional and prospective study of exercise and depressed mood in the
elderly : the Rancho Bernardo study. Am.J.Epidemiol. 2001 Mar
15;153(6):596-603.
- Juarbe TC, Gutierrez Y, Gilliss C, Lee KA.
Depressive symptoms, physical activity, and weight gain in premenopausal Latina
and White women. Maturitas 2006 Sep 20;55(2):116-25.
- Knox S, Barnes A, Kiefe C, Lewis CE, Iribarren C,
Matthews KA, Wong ND, Whooley M. History of depression, race, and
cardiovascular risk in CARDIA. Int.J.Behav.Med. 2006;13(1):44-50.
- Rubin RR, Knowler WC, Ma Y, Marrero DG, Edelstein
SL, Walker EA, Garfield SA, Fisher EB. Depression symptoms and antidepressant
medicine use in Diabetes Prevention Program participants. Diabetes Care 2005
Apr;28(4):830-7.
- Walker LO, Freeland-Graves JH, Milani T,
Hanss-Nuss H, George G, Sterling BS, Kim M, Timmerman GM, Wilkinson S, Arheart
KL, et al. Weight and behavioral and psychosocial factors among ethnically
diverse, low-income women after childbirth: I. Methods and context. Women
Health 2004;40(2):1-17.
- Kiernan M, King AC, Stefanick ML, Killen JD. Men
gain additional psychological benefits by adding exercise to a weight-loss
program. Obes.Res. 2001 Dec;9(12):770-7.
- Anderson RT, King A, Stewart AL, Camacho F,
Rejeski WJ. Physical activity counseling in primary care and patient
well-being: Do patients benefit? Ann.Behav.Med. 2005 Oct;30(2):146-54.
- Gary R, Lee SY. Physical function and quality of
life in older women with diastolic heart failure: effects of a progressive
walking program on sleep patterns. Prog.Cardiovasc.Nurs. 2007;22(2):72-80.
- Neidig JL, Smith BA, Brashers DE. Aerobic
exercise training for depressive symptom management in adults living with HIV
infection. J.Assoc.Nurses AIDS Care 2003 Mar;14(2):30-40.
- Neuberger GB, Aaronson LS, Gajewski B, Embretson
SE, Cagle PE, Loudon JK, Miller PA. Predictors of exercise and effects of
exercise on symptoms, function, aerobic fitness, and disease outcomes of
rheumatoid arthritis. Arthritis Rheum. 2007 Aug 15;57(6):943-52.
- Segar ML, Katch VL, Roth RS, Garcia AW, Portner
TI, Glickman SG, Haslanger S, Wilkins EG. The effect of aerobic exercise on
self-esteem and depressive and anxiety symptoms among breast cancer survivors.
Oncol.Nurs.Forum 1998 Jan;25(1):107-13.
- Damush TM, Damush JG, Jr. The effects of strength
training on strength and health-related quality of life in older adult women.
Gerontologist 1999 Dec;39(6):705-10.
- Dugmore LD, Tipson RJ, Phillips MH, Flint EJ,
Stentiford NH, Bone MF, Littler WA. Changes in cardiorespiratory fitness,
psychological wellbeing, quality of life, and vocational status following a 12
month cardiac exercise rehabilitation programme. Heart 1999
Apr;81(4):359-66.
- Koukouvou G, Kouidi E, Iacovides A,
Konstantinidou E, Kaprinis G, Deligiannis A. Quality of life, psychological and
physiological changes following exercise training in patients with chronic
heart failure. J.Rehabil.Med. 2004 Jan;36(1):36-41.
- Seki E, Watanabe Y, Sunayama S, Iwama Y, Shimada
K, Kawakami K, Sato M, Sato H, Mokuno H, Daida H. Effects of phase III cardiac
rehabilitation programs on health-related quality of life in elderly patients
with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP).
Circ.J. 2003 Jan;67(1):73-7.
- Bernaards CM, Jans MP, van den Heuvel SG,
Hendriksen IJ, Houtman IL, Bongers PM. Can strenuous leisure time physical
activity prevent psychological complaints in a working population?
Occup.Environ.Med. 2006 Jan;63(1):10-6.
- Brown WJ, Ford JH, Burton NW, Marshall AL, Dobson
AJ. Prospective study of physical activity and depressive symptoms in
middle-aged women. Am.J.Prev.Med. 2005 Nov;29(4):265-72.
- Lampinen P, Heikkinen RL, Ruoppila I. Changes in
intensity of physical exercise as predictors of depressive symptoms among older
adults: an eight-year follow-up. Prev.Med. 2000 May;30(5):371-80.
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN,
Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and
public health: updated recommendation for adults from the American College of
Sports Medicine and the American Heart Association. Circulation 2007 Aug
28;116(9):1081-93.
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera
CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, et al. Physical
activity and public health. A recommendation from the Centers for Disease
Control and Prevention and the American College of Sports Medicine. JAMA 1995
Feb 1;273(5):402-7.
- Assis MR, Silva LE, Alves AM, Pessanha AP, Valim
V, Feldman D, Neto TL, Natour J. A randomized controlled trial of deep water
running: clinical effectiveness of aquatic exercise to treat fibromyalgia.
Arthritis Rheum. 2006 Feb 15;55(1):57-65.
- Arnardottir RH, Boman G, Larsson K, Hedenstrom H,
Emtner M. Interval training compared with continuous training in patients with
COPD. Respir.Med. 2007 Jun;101(6):1196-204.
- Cheung BM, Lo JL, Fong DY, Chan MY, Wong SH, Wong
VC, Lam KS, Lau CP, Karlberg JP. Randomised controlled trial of qigong in the
treatment of mild essential hypertension. J.Hum.Hypertens. 2005
Sep;19(9):697-704.
- Palmer JA, Palmer LK, Michiels K, Thigpen B.
Effects of type of exercise on depression in recovering substance abusers.
Percept.Mot.Skills 1995 Apr;80(2):523-30.
- Puhan MA, Busching G, Schunemann HJ, VanOort E,
Zaugg C, Frey M. Interval versus continuous high-intensity exercise in chronic
obstructive pulmonary disease: a randomized trial. Ann.Intern.Med. 2006 Dec
5;145(11):816-25.
- Tsang HW, Chan EP. Effects of mindful and
non-mindful exercises on people with depression: A systematic review.
Br.J.Clin.Psychol. 2008 Jan 31.
- Kessler RC, Gruber M, Hettema JM, Hwang I,
Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety
disorders in the National Comorbidity Survey follow-up. Psychol.Med. 2007 Nov
30;1-10.
- DuPont RL, Rice DP, Miller LS, Shiraki SS,
Rowland CR, Harwood HJ. Economic costs of anxiety disorders. Anxiety.
1996;2(4):167-72.
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein
SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of
anxiety disorders in the 1990s. J.Clin.Psychiatry 1999 Jul;60(7):427-35.
- De Moor MH, Beem AL, Stubbe JH, Boomsma DI, De
Geus EJ. Regular exercise, anxiety, depression and personality: a
population-based study. Prev.Med. 2006 Apr;42(4):273-9.
- Strine TW, Chapman DP, Kobau R, Balluz L.
Associations of self-reported anxiety symptoms with health-related quality of
life and health behaviors. Soc.Psychiatry Psychiatr.Epidemiol. 2005
Jun;40(6):432-8.
- Thorsen L, Nystad W, Stigum H, Dahl O, Klepp O,
Bremnes RM, Wist E, Fossa SD. The association between self-reported physical
activity and prevalence of depression and anxiety disorder in long-term
survivors of testicular cancer and men in a general population sample.
Support.Care Cancer 2005 Aug;13(8):637-46.
- Broocks A, Bandelow B, Pekrun G, George A, Meyer
T, Bartmann U, Hillmer-Vogel U, Ruther E. Comparison of aerobic exercise,
clomipramine, and placebo in the treatment of panic disorder. Am.J.Psychiatry
1998 May;155(5):603-9.
- Merom D, Phongsavan P, Wagner R, Chey T, Marnane
C, Steel Z, Silove D, Bauman A. Promoting walking as an adjunct intervention to
group cognitive behavioral therapy for anxiety disorders-A pilot group
randomized trial. J.Anxiety.Disord. 2007 Sep 29.
- Long BC, van Stavel R. Effects of exercise
training on anxiety: a meta-analysis. Journal of Applied Sport Psychology
1995;7(2):167-89.
- Hassmen P, Koivula N. Mood, physical working
capacity and cognitive performance in the elderly as related to physical
activity. Aging (Milano.) 1997 Feb;9(1-2):136-42.
- Blumenthal JA, Sherwood A, Babyak MA, Watkins LL,
Waugh R, Georgiades A, Bacon SL, Hayano J, Coleman RE, Hinderliter A. Effects
of exercise and stress management training on markers of cardiovascular risk in
patients with ischemic heart disease: a randomized controlled trial. JAMA 2005
Apr 6;293(13):1626-34.
- Brown MA, Goldstein-Shirley J, Robinson J, Casey
S. The effects of a multi-modal intervention trial of light, exercise, and
vitamins on women's mood. Women Health 2001;34(3):93-112.
- Castro CM, Wilcox S, O'Sullivan P, Baumann K,
King AC. An exercise program for women who are caring for relatives with
dementia. Psychosom.Med. 2002 May;64(3):458-68.
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann
JA, Haisfield-Wolfe ME, Quitasol W, Mitchell S, Chakravarthy A, Gage I. Effects
of exercise on fatigue, physical functioning, and emotional distress during
radiation therapy for breast cancer. Oncol.Nurs.Forum 1997
Jul;24(6):991-1000.
- Schachter CL, Busch AJ, Peloso PM, Sheppard MS.
Effects of short versus long bouts of aerobic exercise in sedentary women with
fibromyalgia: a randomized controlled trial. Phys.Ther. 2003
Apr;83(4):340-58.
- Tsutsumi T, Don BM, Zaichkowsky LD, Takenaka K,
Oka K, Ohno T. Comparison of high and moderate intensity of strength training
on mood and anxiety in older adults. Percept.Mot.Skills 1998 Dec;87(3 Pt
1):1003-11.
- Backmand H, Kaprio J, Kujala U, Sarna S.
Influence of physical activity on depression and anxiety of former elite
athletes. Int.J.Sports Med. 2003 Nov;24(8):609-19.
- Annesi J. Effects of cardiovascular exercise
frequency and duration on depression and tension changes over 10 weeks.
Eur.J.Sport Sci. 2003;3(4):1-12.
- Osei-Tutu KB, Campagna PD. The effects of short-
vs. long-bout exercise on mood, VO2max, and percent body fat. Prev.Med. 2005
Jan;40(1):92-8.
- Cassilhas RC, Viana VA, Grassmann V, Santos RT,
Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the
cognitive function of the elderly. Med.Sci.Sports Exerc. 2007
Aug;39(8):1401-7.
- Gill SC, Butterworth P, Rodgers B, Mackinnon A.
Validity of the mental health component scale of the 12-item Short-Form Health
Survey (MCS-12) as measure of common mental disorders in the general
population. Psychiatry Res. 2007 Jul 30;152(1):63-71.
- Kessler RC, Andrews G, Colpe LJ, Hiripi E,
Mroczek DK, Normand SL, Walters EE, Zaslavsky AM. Short screening scales to
monitor population prevalences and trends in non-specific psychological
distress. Psychol.Med. 2002 Aug;32(6):959-76.
- Stansfeld SA, Marmot MG. Stress and the heart:
psychosocial pathways to coronary heart disease. London: BMJ Books; 2002.
- Pressman SD, Cohen S. Does positive affect
influence health? Psychol.Bull. 2005 Nov;131(6):925-71.
- Stewart AL, Hays RD, Wells KB, Rogers WH,
Spritzer KL, Greenfield S. Long-term functioning and well-being outcomes
associated with physical activity and exercise in patients with chronic
conditions in the Medical Outcomes Study. J.Clin.Epidemiol. 1994
Jul;47(7):719-30.
- Kruger J, Bowles HR, Jones DA, Ainsworth BE, Kohl
HW, III. Health-related quality of life, BMI and physical activity among US
adults (>/=18 years): National Physical Activity and Weight Loss Survey,
2002. Int.J.Obes.(Lond) 2007 Feb;31(2):321-7.
- Shih M, Hootman JM, Kruger J, Helmick CG.
Physical activity in men and women with arthritis National Health Interview
Survey, 2002. Am.J.Prev.Med. 2006 May;30(5):385-93.
- Strine TW, Hootman JM, Okoro CA, Balluz L,
Moriarty DG, Owens M, Mokdad A. Frequent mental distress status among adults
with arthritis age 45 years and older, 2001. Arthritis Rheum. 2004 Aug
15;51(4):533-7.
- Wolin KY, Glynn RJ, Colditz GA, Lee IM, Kawachi
I. Long-term physical activity patterns and health-related quality of life in
U.S. women. Am.J.Prev.Med. 2007 Jun;32(6):490-9.
- Zahran HS, Zack MM, Vernon-Smiley ME, Hertz MF.
Health-related quality of life and behaviors risky to health among adults aged
18-24 years in secondary or higher education--United States, 2003-2005.
J.Adolesc.Health 2007 Oct;41(4):389-97.
- Bhui K, Fletcher A. Common mood and anxiety
states: gender differences in the protective effect of physical activity.
Soc.Psychiatry Psychiatr.Epidemiol. 2000 Jan;35(1):28-35.
- Bultmann U, Kant IJ, Kasl SV, Schroer KA, Swaen
GM, van den Brandt PA. Lifestyle factors as risk factors for fatigue and
psychological distress in the working population: prospective results from the
Maastricht Cohort Study. J.Occup.Environ.Med. 2002 Feb;44(2):116-24.
- Schnohr P, Kristensen TS, Prescott E, Scharling
H. Stress and life dissatisfaction are inversely associated with jogging and
other types of physical activity in leisure time--The Copenhagen City Heart
Study. Scand.J.Med.Sci.Sports 2005 Apr;15(2):107-12.
- Wendel-Vos GC, Schuit AJ, Tijhuis MA, Kromhout D.
Leisure time physical activity and health-related quality of life:
cross-sectional and longitudinal associations. Qual.Life Res. 2004
Apr;13(3):667-77.
- Basen-Engquist K, Taylor CL, Rosenblum C, Smith
MA, Shinn EH, Greisinger A, Gregg X, Massey P, Valero V, Rivera E. Randomized
pilot test of a lifestyle physical activity intervention for breast cancer
survivors. Patient.Educ.Couns. 2006 Dec;64(1-3):225-34.
- Wiles NJ, Haase AM, Gallacher J, Lawlor DA,
Lewis G. Physical activity and common mental disorder: results from the
Caerphilly study. Am.J.Epidemiol. 2007 Apr 15;165(8):946-54.
- Lezak MD, Howieson DB, Loring DW.
Neuropsychological assessment. 4th ed. Oxford; New York: Oxford University
Press; 2004.
- Dik M, Deeg DJ, Visser M, Jonker C. Early life
physical activity and cognition at old age. J.Clin.Exp.Neuropsychol. 2003
Aug;25(5):643-53.
- Flicker L, Almeida OP, Acres J, Le MT, Tuohy RJ,
Jamrozik K, Hankey G, Norman P. Predictors of impaired cognitive function in
men over the age of 80 years: results from the Health in Men Study. Age Ageing
2005 Jan;34(1):77-80.
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware
JH, Grodstein F. Physical activity, including walking, and cognitive function
in older women. JAMA 2004 Sep 22;292(12):1454-61.
- Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K.
A prospective study of physical activity and cognitive decline in elderly
women: women who walk. Arch.Intern.Med. 2001 Jul 23;161(14):1703-8.
- Broe GA, Creasey H, Jorm AF, Bennett HP, Casey
B, Waite LM, Grayson DA, Cullen J. Health habits and risk of cognitive
impairment and dementia in old age: a prospective study on the effects of
exercise, smoking and alcohol consumption. Aust.N.Z.J.Public Health 1998
Aug;22(5):621-3.
- Sturman MT, Morris MC, Mendes de Leon CF,
Bienias JL, Wilson RS, Evans DA. Physical activity, cognitive activity, and
cognitive decline in a biracial community population. Arch.Neurol. 2005
Nov;62(11):1750-4.
- Rovio S, Kareholt I, Helkala EL, Viitanen M,
Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M. Leisure-time
physical activity at midlife and the risk of dementia and Alzheimer's disease.
Lancet Neurol. 2005 Nov;4(11):705-11.
- Larson EB, Wang L, Bowen JD, McCormick WC, Teri
L, Crane P, Kukull W. Exercise is associated with reduced risk for incident
dementia among persons 65 years of age and older. Ann.Intern.Med. 2006 Jan
17;144(2):73-81.
- Colcombe S, Kramer AF. Fitness effects on the
cognitive function of older adults: a meta-analytic study. Psychol.Sci. 2003
Mar;14(2):125-30.
- Ozkaya GY, Aydin H, Toraman FN, Kizilay F,
Ozdemir O, Cetinkaya V. Effect of strength and endurance training on cognition
in older people. Journal of Sports Science and Medicine 2005
Sep;4(3):300-13.
- Oken BS, Zajdel D, Kishiyama S, Flegal K, Dehen
C, Haas M, Kraemer DF, Lawrence J, Leyva J. Randomized, controlled, six-month
trial of yoga in healthy seniors: effects on cognition and quality of life.
Altern.Ther.Health Med. 2006 Jan;12(1):40-7.
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of
exercise training on elderly persons with cognitive impairment and dementia: a
meta-analysis. Arch.Phys.Med.Rehabil. 2004 Oct;85(10):1694-704.
- Scherder EJ, Van PJ, Deijen JB, Van Der KS,
Orlebeke JF, Burgers I, Devriese PP, Swaab DF, Sergeant JA. Physical activity
and executive functions in the elderly with mild cognitive impairment. Aging
Ment.Health 2005 May;9(3):272-80.
- Stevens J, Killeen M. A randomised controlled
trial testing the impact of exercise on cognitive symptoms and disability of
residents with dementia. Contemp.Nurse 2006 Feb;21(1):32-40.
- Podewils LJ, Guallar E, Kuller LH, Fried LP,
Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and
dementia risk: findings from the Cardiovascular Health Cognition Study.
Am.J.Epidemiol. 2005 Apr 1;161(7):639-51.
- Schuit AJ, Feskens EJ, Launer LJ, Kromhout D.
Physical activity and cognitive decline, the role of the apolipoprotein e4
allele. Med.Sci.Sports Exerc. 2001 May;33(5):772-7.
- Simons LA, Simons J, McCallum J, Friedlander Y.
Lifestyle factors and risk of dementia: Dubbo Study of the elderly. Med.J.Aust.
2006 Jan 16;184(2):68-70.
- Eggermont L, Swaab D, Luiten P, Scherder E.
Exercise, cognition and Alzheimer's disease: more is not necessarily better.
Neurosci.Biobehav.Rev. 2006;30(4):562-75.
- Salthouse TA, Davis HP. Organization of
cognitive abilities and neuropsychological variables across the lifespan.
Developmental Review 2006 Mar;26(1):31-54.
- Abbott RD, White LR, Ross GW, Masaki KH, Curb
JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA
2004 Sep 22;292(12):1447-53.
- Laurin D, Verreault R, Lindsay J, MacPherson K,
Rockwood K. Physical activity and risk of cognitive impairment and dementia in
elderly persons. Arch.Neurol. 2001 Mar;58(3):498-504.
- Wang L, van BG, Kukull WB, Larson EB. Predictors
of functional change: a longitudinal study of nondemented people aged 65 and
older. J.Am.Geriatr.Soc. 2002 Sep;50(9):1525-34.
- Wilson RS, Bennett DA, Bienias JL, Aggarwal NT,
Mendes de Leon CF, Morris MC, Schneider JA, Evans DA. Cognitive activity and
incident AD in a population-based sample of older persons. Neurology 2002 Dec
24;59(12):1910-4.
- Barnes DE, Yaffe K, Satariano WA, Tager IB. A
longitudinal study of cardiorespiratory fitness and cognitive function in
healthy older adults. J.Am.Geriatr.Soc. 2003 Apr;51(4):459-65.
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P,
McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular
fitness, cortical plasticity, and aging. Proc.Natl.Acad.Sci.U.S.A 2004 Mar
2;101(9):3316-21.
- Kramer AF, Erickson KI, Colcombe SJ. Exercise,
cognition, and the aging brain. J.Appl.Physiol 2006 Oct;101(4):1237-42.
- Marks BL, Madden DJ, Bucur B, Provenzale JM,
White LE, Cabeza R, Huettel SA. Role of aerobic fitness and aging on cerebral
white matter integrity. Ann.N.Y.Acad.Sci. 2007 Feb;1097:171-4.
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen
NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging
humans. J.Gerontol.A Biol.Sci.Med.Sci. 2003 Feb;58(2):176-80.
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS,
Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise
training increases brain volume in aging humans. J.Gerontol.A Biol.Sci.Med.Sci.
2006 Nov;61(11):1166-70.
- Pereira AC, Huddleston DE, Brickman AM, Sosunov
AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo
correlate of exercise-induced neurogenesis in the adult dentate gyrus.
Proc.Natl.Acad.Sci.U.S.A 2007 Mar 27;104(13):5638-43.
- Etnier JL, Nowell PM, Landers DM, Sibley BA. A
meta-regression to examine the relationship between aerobic fitness and
cognitive performance. Brain Res.Rev. 2006 Aug 30;52(1):119-30.
- National Center on Sleep Disorders Research
(National Heart LaBI. Frontiers of knowledge in sleep & sleep disorders:
opportunities for improving health and quality of life 2004 Available
from http://videocast.nih.gov/launch.asp?11949.
- Youngstedt SD, Kline CE. Epidemiology of
exercise and sleep. Sleep and Biological Rhythms 2006;4(3):215-21.
- Morgan K. Daytime activity and risk factors for
late-life insomnia. J.Sleep Res. 2003 Sep;12(3):231-8.
- Akerstedt T, Knutsson A, Westerholm P, Theorell
T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: a
cross-sectional study. J.Psychosom.Res. 2002 Sep;53(3):741-8.
- Bazargan M. Self-reported sleep disturbance
among African-American elderly: the effects of depression, health status,
exercise, and social support. Int.J.Aging Hum.Dev. 1996;42(2):143-60.
- Hublin C, Kaprio J, Partinen M, Koskenvuo M.
Insufficient sleep--a population-based study in adults. Sleep 2001 Jun
15;24(4):392-400.
- Kawamoto R, Yoshida O, Oka Y, Takagi Y. Risk
factors for insomnia in community-dwelling older persons. Geriatrics &
Gerontology International 2004 Sep;4(3):163-8.
- Kim K, Uchiyama M, Okawa M, Liu X, Ogihara R. An
epidemiological study of insomnia among the Japanese general population. Sleep
2000 Feb 1;23(1):41-7.
- Kravitz HM, Ganz PA, Bromberger J, Powell LH,
Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community
survey of sleep and the menopausal transition. Menopause. 2003
Jan;10(1):19-28.
- Ohayon MM. Interactions between sleep normative
data and sociocultural characteristics in the elderly. J.Psychosom.Res. 2004
May;56(5):479-86.
- Ohida T, Kamal AM, Uchiyama M, Kim K, Takemura
S, Sone T, Ishii T. The influence of lifestyle and health status factors on
sleep loss among the Japanese general population. Sleep 2001 May
1;24(3):333-8.
- Phillips B, Young T, Finn L, Asher K, Hening WA,
Purvis C. Epidemiology of restless legs symptoms in adults. Arch.Intern.Med.
2000 Jul 24;160(14):2137-41.
- Sherrill DL, Kotchou K, Quan SF. Association of
physical activity and human sleep disorders. Arch.Intern.Med. 1998 Sep
28;158(17):1894-8.
- Peppard PE, Young T. Exercise and
sleep-disordered breathing: an association independent of body habitus. Sleep
2004 May 1;27(3):480-4.
- Quan SF, O'Connor GT, Quan JS, Redline S,
Resnick HE, Shahar E, Siscovick D, Sherrill DL. Association of physical
activity with sleep-disordered breathing. Sleep Breath. 2007
Sep;11(3):149-57.
- Guilleminault C, Clerk A, Black J, Labanowski M,
Pelayo R, Claman D. Nondrug treatment trials in psychophysiologic insomnia.
Arch.Intern.Med. 1995 Apr 24;155(8):838-44.
- King AC, Oman RF, Brassington GS, Bliwise DL,
Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in
older adults. A randomized controlled trial. JAMA 1997 Jan 1;277(1):32-7.
- King AC, Baumann K, O'Sullivan P, Wilcox S,
Castro C. Effects of moderate-intensity exercise on physiological, behavioral,
and emotional responses to family caregiving: a randomized controlled trial.
J.Gerontol.A Biol.Sci.Med.Sci. 2002 Jan;57(1):M26-M36.
- Tworoger SS, Yasui Y, Vitiello MV, Schwartz RS,
Ulrich CM, Aiello EJ, Irwin ML, Bowen D, Potter JD, McTiernan A. Effects of a
yearlong moderate-intensity exercise and a stretching intervention on sleep
quality in postmenopausal women. Sleep 2003 Nov 1;26(7):830-6.
- Youngstedt SD, O'Connor PJ, Dishman RK. The
effects of acute exercise on sleep: a quantitative synthesis. Sleep 1997
Mar;20(3):203-14.
- Youngstedt SD. Effects of exercise on sleep.
Clin.Sports Med. 2005 Apr;24(2):355-65, xi.
- Alessi CA, Schnelle JF, MacRae PG, Ouslander JG,
al-Samarrai N, Simmons SF, Traub S. Does physical activity improve sleep in
impaired nursing home residents? J.Am.Geriatr.Soc. 1995
Oct;43(10):1098-102.
- Alessi CA, Yoon EJ, Schnelle JF, Al-Samarrai NR,
Cruise PA. A randomized trial of a combined physical activity and environmental
intervention in nursing home residents: do sleep and agitation improve?
J.Am.Geriatr.Soc. 1999 Jul;47(7):784-91.
- Alessi CA, Martin JL, Webber AP, Cynthia KE,
Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological
intervention to improve abnormal sleep/wake patterns in nursing home residents.
J.Am.Geriatr.Soc. 2005 May;53(5):803-10.
- Ouslander JG, Connell BR, Bliwise DL, Endeshaw
Y, Griffiths P, Schnelle JF. A nonpharmacological intervention to improve sleep
in nursing home patients: results of a controlled clinical trial.
J.Am.Geriatr.Soc. 2006 Jan;54(1):38-47.
- Yamamoto U, Mohri M, Shimada K, Origuchi H,
Miyata K, Ito K, Abe K, Yamamoto H. Six-month aerobic exercise training
ameliorates central sleep apnea in patients with chronic heart failure. J.Card
Fail. 2007 Dec;13(10):825-9.
- Singh NA, Clements KM, Fiatarone MA. A
randomized controlled trial of the effect of exercise on sleep. Sleep 1997
Feb;20(2):95-101.
- de Jong J, Lemmink KA, Stevens M, de Greef MH,
Rispens P, King AC, Mulder T. Six-month effects of the Groningen active living
model (GALM) on physical activity, health and fitness outcomes in sedentary and
underactive older adults aged 55-65. Patient.Educ.Couns. 2006
Jul;62(1):132-41.
- Elavsky S, McAuley E. Lack of perceived sleep
improvement after 4-month structured exercise programs. Menopause. 2007
May;14(3 Pt 1):535-40.
- Yurtkuran M, Alp A, Yurtkuran M, Dilek K. A
modified yoga-based exercise program in hemodialysis patients: a randomized
controlled study. Complement Ther.Med. 2007 Sep;15(3):164-71.
- Motl RW, Gosney JL. Effect of exercise training
on quality of life in multiple sclerosis: a meta-analysis. Mult.Scler. 2008
Jan;14(1):129-35.
- Goodwin VA, Richards SH, Taylor RS, Taylor AH,
Campbell JL. The effectiveness of exercise interventions for people with
Parkinson's disease: A systematic review and meta-analysis. Mov Disord. 2008
Jan 7.
- Spence JC, McGannon JR, Poon P. The effect of
exercise on global self-esteem: a quantitative review. Journal of Sport
& Exercise Psychology 2005;27(3):311-34.
- Fulcher KY, White PD. Randomised controlled
trial of graded exercise in patients with the chronic fatigue syndrome. BMJ
1997 Jun 7;314(7095):1647-52.
- Moss-Morris R, Sharon C, Tobin R, Baldi JC. A
randomized controlled graded exercise trial for chronic fatigue syndrome:
outcomes and mechanisms of change. J.Health Psychol. 2005
Mar;10(2):245-59.
- Powell P, Bentall RP, Nye FJ, Edwards RH.
Randomised controlled trial of patient education to encourage graded exercise
in chronic fatigue syndrome. BMJ 2001 Feb 17;322(7283):387-90.
- Wallman KE, Morton AR, Goodman C, Grove R,
Guilfoyle AM. Randomised controlled trial of graded exercise in chronic fatigue
syndrome. Med.J.Aust. 2004 May 3;180(9):444-8.
- Wearden AJ, Morriss RK, Mullis R, Strickland PL,
Pearson DJ, Appleby L, Campbell IT, Morris JA. Randomised, double-blind,
placebo-controlled treatment trial of fluoxetine and graded exercise for
chronic fatigue syndrome. Br.J.Psychiatry 1998 Jun;172:485-90.
- Puetz TW. Physical activity and feelings of
energy and fatigue: epidemiological evidence. Sports Med.
2006;36(9):767-80.
- Puetz TW, Flowers SS, O'Connor PJ. A randomized
controlled trial of the effect of aerobic exercise training on feelings of
energy and fatigue in sedentary young adults with persistent fatigue.
Psychother.Psychosom. 2008 Feb 14;77(3):167-74.
- Puetz TW, O'Connor PJ, Dishman RK. Effects of
chronic exercise on feelings of energy and fatigue: a quantitative synthesis.
Psychol.Bull. 2006 Nov;132(6):866-76.
- Morgan WP. Negative addiction in runners.
Physician and Sportmedicine 1979;7(2):57-70.
- Dishman RK. Physical activity and mental health.
In: Friedman HS, editor. The Encyclopedia of Mental Health. San Diego: Academic
Press; 1998.
- Davis C. Exercise abuse. International Journal
of Sport Psychology 2000;31(2):278-89.
- Sundgot-Borgen J. Eating disorders in female
athletes. Sports Med. 1994 Mar;17(3):176-88.
- O'Connor PJ, Smith JC. Physical activity and
eating disorders. In: Rippe JM, editor. Lifestyle Medicine. Malden, MA:
Blackwell Science, Inc.; 1999. p. 1005-15.
- Gruber AJ, Pope HG, Jr. Psychiatric and medical
effects of anabolic-androgenic steroid use in women. Psychother.Psychosom.
2000;69(1):19-26.
- Pope HG, Jr., Gruber AJ, Choi P, Olivardia R,
Phillips KA. Muscle dysmorphia. An underrecognized form of body dysmorphic
disorder. Psychosomatics 1997 Nov;38(6):548-57.
- Koltyn KF, Lynch NA, Hill DW. Psychological
responses to brief exhaustive cycling exercise in the morning and the evening.
Int.J.Sport Psychol. 1998 Apr;29(2):145-56.
- O'Connor PJ, Petruzzello SJ, Kubitz KA, Robinson
TL. Anxiety responses to maximal exercise testing. Br.J.Sports Med. 1995
Jun;29(2):97-102.
- O'Connor PJ, Smith JC, Morgan WP. Physical
activity does not provoke panic attacks in patients with panic disorder:
a review of the evidence. Anxiety, Stress, and Coping 2000;13(4):333-53.
- Martinsen EW, Raglin JS, Hoffart A, Friis S.
Tolerance to intensive exercise and high levels of lactate in panic disorder.
J.Anxiety.Disord. 1998 Jul;12(4):333-42.
- Garvin AW, Koltyn KF, Morgan WP. Influence of
acute physical activity and relaxation on state anxiety and blood lactate in
untrained college males. Int.J.Sports Med. 1997 Aug;18(6):470-6.
- Dishman RK, Berthoud HR, Booth FW, Cotman CW,
Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman
CH, et al. Neurobiology of exercise. Obesity.(Silver.Spring) 2006
Mar;14(3):345-56.
- Dishman RK, Dunn AL, Youngstedt SD, Davis JM,
Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased
striatal GABAA binding after activity wheel running. Physiol Behav. 1996
Sep;60(3):699-705.
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG,
Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running
reduces escape latency and alters brain monoamine levels after footshock. Brain
Res.Bull. 1997;42(5):399-406.
- Greenwood BN, Strong PV, Dorey AA, Fleshner M.
Therapeutic effects of exercise: wheel running reverses stress-induced
interference with shuttle box escape. Behav.Neurosci. 2007
Oct;121(5):992-1000.
- Hunsberger JG, Newton SS, Bennett AH, Duman CH,
Russell DS, Salton SR, Duman RS. Antidepressant actions of the
exercise-regulated gene VGF. Nat.Med. 2007 Dec;13(12):1476-82.
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman
CW. Physical activity and antidepressant treatment potentiate the expression of
specific brain-derived neurotrophic factor transcripts in the rat hippocampus.
Neuroscience 2000;101(2):305-12.
- Van Hoomissen JD, Holmes PV, Zellner AS,
Poudevigne A, Dishman RK. Effects of beta-adrenoreceptor blockade during
chronic exercise on contextual fear conditioning and mRNA for galanin and
brain-derived neurotrophic factor. Behav.Neurosci. 2004
Dec;118(6):1378-90.
- Yoo HS, Tackett RL, Bunnell BN, Crabbe JB,
Dishman RK. Antidepressant-like effects of physical activity versus
imipramine: neonatal clomipramine model. Psychobiology
2000;28(4):540-9.
- Knight JA, Thompson S, Raboud JM, Hoffman BR.
Light and exercise and melatonin production in women. Am.J.Epidemiol. 2005 Dec
1;162(11):1114-22.
- Van Reeth O, Sturis J, Byrne MM, Blackman JD,
L'Hermite-Baleriaux M, Leproult R, Oliner C, Refetoff S, Turek FW, Van CE.
Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin
secretion in normal men. Am.J.Physiol 1994 Jun;266(6 Pt 1):E964-E974.
- Barger LK, Wright KP, Jr., Hughes RJ, Czeisler
CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in
very dim light. Am.J.Physiol Regul.Integr.Comp Physiol 2004
Jun;286(6):R1077-R1084.
- Benington JH, Kodali SK, Heller HC. Stimulation
of A1 adenosine receptors mimics the electroencephalographic effects of sleep
deprivation. Brain Res. 1995 Sep 18;692(1-2):79-85.
- Motl RW, Konopack JF, McAuley E, Elavsky S,
Jerome GJ, Marquez DX. Depressive symptoms among older adults: long-term
reduction after a physical activity intervention. J.Behav.Med. 2005
Aug;28(4):385-94.
- Bartholomew JB, Morrison D, Ciccolo JT. Effects
of acute exercise on mood and well-being in patients with major depressive
disorder. Med.Sci.Sports Exerc. 2005 Dec;37(12):2032-7.
- O'Connor PJ, Raglin JS, Martinsen EW. Physical
activity, anxiety, and anxiety disorders. Int.J.Sport Psychol.
2000;31(2):136-55.
- Lara A, Yancey AK, Tapia-Conye R, Flores Y,
Kuri-Morales P, Mistry R, Subirats E, McCarthy WJ. Pausa para tu Salud:
reduction of weight and waistlines by integrating exercise breaks into
workplace organizational routine. Prev.Chronic.Dis. 2008 Jan;5(1):A12.
- Pronk SJ, Pronk NP, Sisco A, Ingalls DS, Ochoa
C. Impact of a daily 10-minute strength and flexibility program in a
manufacturing plant. Am.J.Health Promot. 1995 Jan;9(3):175-8.
- Yancey AK, Fielding JE, Flores GR, Sallis JF,
McCarthy WJ, Breslow L. Creating a robust public health infrastructure for
physical activity promotion. Am.J.Prev.Med. 2007 Jan;32(1):68-78.
- Yancey AK, Lewis LB, Guinyard JJ, Sloane DC,
Nascimento LM, Galloway-Gilliam L, Diamant AL, McCarthy WJ. Putting promotion
into practice: the African Americans building a legacy of health organizational
wellness program. Health Promot.Pract. 2006 Jul;7(3 Suppl):233S-46S.
- Yancey AK, McCarthy WJ, Taylor WC, Merlo A, Gewa
C, Weber MD, Fielding JE. The Los Angeles Lift Off: a sociocultural
environmental change intervention to integrate physical activity into the
workplace. Prev.Med. 2004 Jun;38(6):848-56.
top of page
Continue to G9. Youth
Back to G7. Cancer
Back to Physical Activity Guidelines Advisory Committee Report |