


   |
| FDA Home Page | CDRH Home Page | Search | A-Z Index |  |
|
 |
 |
||||
 LUMA™ Cervical Imaging System - P040028
LUMA™ Cervical Imaging System - P040028This is a brief overview of information related to FDA's approval to market this product. See the links below to the Summary of Safety and Effectiveness and product labeling for more complete information on this product, its indications for use, and the basis for FDA's approval.
Product Name: LUMA™ Cervical Imaging System
PMA Applicant: MediSpectra, Inc.
Address: 45 Hartwell Avenue Lexington, MA 02421
Approval Date: March 16, 2006
Approval Letter: http://www.fda.gov/cdrh/pdf4/p040028a.pdf
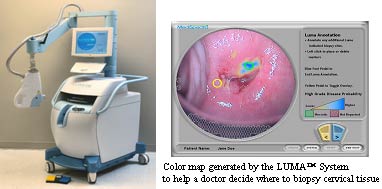
What is it? The LUMA™ Cervical Imaging System is an optical detection system that helps the doctor identify areas on the cervix that may have disease (i.e., areas that are likely to contain precancerous cells). The doctor uses this device immediately after colposcopy to decide where to take additional tissue samples (biopsies).
How does it work? The LUMA™ Cervical Imaging System shines a light on the cervix and analyzes how different areas of the cervix respond to this light. The LUMA System assigns a score to tiny areas of the cervix and produces a color map that helps the doctor decide where to biopsy. The colors and patterns on the map help the doctor distinguish between healthy tissue, potentially diseased tissue, and areas that cannot be interpreted (e.g. tissue at the edge of the screen).
When is it used? The LUMA™ Cervical Imaging System is used immediately after a procedure called colposcopy, a high magnification evaluation of the cervix for women who have recently had an abnormal Pap smear. The doctor first performs colposcopy and identifies areas on the cervix to biopsy. The doctor then evaluates the LUMA™ image to see whether or not there are additional areas of the cervix that should be biopsied. Only after both the colposcopy and LUMA™ procedures are completed does the doctor perform the biopsies indicated from the procedures.
What will it accomplish? The LUMA™ Cervical Imaging System will help improve the chances that the doctor does not miss an area that may contain precancerous cells.
Additional information: The Summary of Safety and Effectiveness and labeling will be available at: http://www.fda.gov/cdrh/pdf4/p040028.html
Other:
Interactive tutorial on colposcopy (requires Flash Player):
http://www.nlm.nih.gov/medlineplus/tutorials/colposcopy/htm/index.htm
Updated March 16, 2006
![]()
CDRH Home Page | CDRH A-Z Index | Contact CDRH | Accessibility | Disclaimer
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA | HHS Home Page
Center for Devices and Radiological Health / CDRH