 |

Digital Mammography Outperforms Film for Some Women
Results from the largest randomized trial ever comparing digital mammography with standard film mammography confirm earlier indications that digital mammography is more accurate for women with dense breasts. Several other groups of women benefited from undergoing screening with digital mammography instead of film, including women under 50 and pre- and perimenopausal women.
Overall, 65 percent of trial participants fell into at least one of these groups.
"We found important breast cancers, the kind that kill women, using digital that we did not find with film, and the difference was significant in all three of those categories," said the study's principal investigator, Dr. Etta Pisano, of the University of North Carolina Department of Radiology. The study was not designed to determine whether there was a mortality benefit from digital compared with film. But, because film mammography has been shown to reduce breast cancer death rates, and digital proved to be more accurate for some women, Dr. Pisano said she "wouldn't be surprised" if it proved to save more lives in the long run.
Read more 



A Catalyst for Change
We have learned a great many things about cancer prevention and control over the past three decades. One of the most definitive messages we have been able to deliver is: Routine mammograms can detect breast cancers at a treatable stage and save lives.
As digital mammography machines matured and entered the clinical realm, questions arose about whether this breast cancer screening method could or should supplant film. Digital mammography can supply images with greater spatial resolution and be manipulated to more clearly define a potentially lethal abnormality in breast tissue. So there was good reason to put digital mammography to the test.
Read more 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

Digital Mammography Outperforms Film for Some Women
Results from the largest randomized trial ever comparing digital mammography with standard film mammography confirm earlier indications that digital mammography is more accurate for women with dense breasts. Several other groups of women benefited from undergoing screening with digital mammography instead of film, including women under 50 and pre- and perimenopausal women.
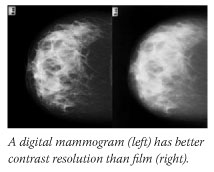 Overall, 65 percent of trial participants fell into at least one of these groups.
Overall, 65 percent of trial participants fell into at least one of these groups.
"We found important breast cancers, the kind that kill women, using digital that we did not find with film, and the difference was significant in all three of those categories," said the study's principal investigator, Dr. Etta Pisano, of the University of North Carolina Department of Radiology. The study was not designed to determine whether there was a mortality benefit from digital compared with film. But, because film mammography has been shown to reduce breast cancer death rates, and digital proved to be more accurate for some women, Dr. Pisano said she "wouldn't be surprised" if it proved to save more lives in the long run.
The nearly 50,000-patient trial - the Digital Mammographic Imaging Screening Trial (DMIST) - was sponsored by the National Cancer Institute (NCI) and conducted by researchers in the American College of Radiology Imaging Network (ACRIN).
Consistent with several smaller trials, overall, the two screening methods performed equally in DMIST. But in the aforementioned subgroups, digital mammography provided a 15- to 28-percent accuracy advantage.
The study results were presented last Friday at an ACRIN meeting in Washington, D.C. - where Dr. Pisano spoke at a news conference - and published on the New England Journal of Medicine Web site.
Study participants underwent mammography by both methods and the results were interpreted independently by two radiologists. Breast cancer status was determined through available breast biopsy information within 15 months of study entry or through follow-up mammography at least 11 months after study entry.
An advantage of digital mammography for women with dense breasts makes sense, explains Dr. Mark Helvie, director of the breast imaging program at the University of Michigan Medical Center.
Dense breasts have a predominance of fibroglandular tissue, which absorbs a lot of x-ray beams and appears white on film. "These white areas can camouflage a cancer, which also looks white," Dr. Helvie says. Digital's superior resolution and image manipulation abilities can help unmask cancers embedded in dense breast tissue.
Although he voiced caution about interpreting too much from one trial, Dr. Helvie agreed that the results "suggest better detection [with digital] if you fall into one of these subsets." He added: "It looks like it's picking up tumors that you would want it to pick up."
Researchers are working on several improvements for digital machines, including ones "that could potentially allow for better spatial resolution," says Dr. Dan Sullivan, director of the NCI Cancer Imaging Program.
Dr. Pisano stressed that only 8 percent of the mammography machines in the United States are digital, meaning that not every woman who the DMIST data suggest should be screened digitally will have access to the technology. Other factors could affect access as well, including the higher cost of digital machines compared with film.
By Carmen Phillips
|
 |
 |

A Catalyst for Change
We have learned a great many things about cancer prevention and control over the past three decades. One of the most definitive messages we have been able to deliver is: Routine mammograms can detect breast cancers at a treatable stage and save lives.
As digital mammography machines matured and entered the clinical realm, questions arose about whether this breast cancer screening method could or should supplant film. Digital mammography can supply images with greater spatial resolution and be manipulated to more clearly define a potentially lethal abnormality in breast tissue. So there was good reason to put digital mammography to the test.
This week's lead story describes the NCI-sponsored DMIST trial, which provides strong evidence that digital mammography is as good as film in the average population and that there is a significant subset of women who benefit from screening with it. Nearly two of three DMIST participants fell into one of the groups in which digital mammography proved more sensitive than standard film mammography, and a large portion of the malignancies caught by digital were of the type that can prove lethal if not detected early.
But there is much more to this story. Beyond its results, DMIST is significant for several other reasons.
The roots of this advance date back to the late 1980s. Digital x-ray machines were being developed for several applications, but because of the technical difficulty of developing devices that had the resolution needed for mammograms, that application was languishing. So in 1991, following the recommendations of an expert consensus conference, NCI issued a Request for Applications (RFA) to speed the development of digital mammography.
The RFA led to the formation of the International Digital Mammography Development Group, an effort that advanced digital mammography to the point where a trial such as DMIST was feasible.
It is particularly noteworthy that NCI and ACRIN secured the involvement of four companies that develop and manufacture digital mammography machines. These four companies are competitors, and their participation was a risk for each in that their equipment might have been shown to be inferior to the others. As it turned out, there was no significant difference demonstrated among the four machines used in DMIST.
DMIST also once again demonstrates NCI's commitment to working with other federal health agencies. Both FDA and the Centers for Medicare and Medicaid Services helped develop the DMIST protocol. Thus, we could ensure that the trial would generate data that would be useful in helping those agencies make regulatory decisions related to digital mammography, which could affect its continued development and availability.
And DMIST is another important addition to the "electronic health care" movement being championed by NCI through efforts such as the cancer Biomedical Informatics Grid. Digital mammogram results will be just one more component of a system in which researchers and clinicians are increasingly becoming virtually connected, sharing their expertise and data to advance cancer prevention, diagnosis, and treatment.
Of course, this trial would not have been possible without the participation of nearly 50,000 women and the dedicated staff at ACRIN and in the NCI Cancer Imaging Program.
Overall, DMIST offers a model case study of how NCI can be an agent of change, pursuing new approaches to research, partnering with the private and public sectors, and fueling the development of technologies to achieve an important payoff.
Dr. Andrew C. von Eschenbach
Director, National Cancer Institute
|
 |
 |

Systems Biology and Cancer
Engineers test their designs for automobiles and airplanes on computers, and similar approaches are being used to study cancer in the emerging field of systems biology.
The field is so new and diverse that systems biology means different things to different people. But clearly one goal is to create mathematical models of biological processes, and this can only be done by integrating information about the components of biological systems, such as genes and proteins, and their environments.
"The essence of systems biology is understanding the interplay between environmental cues and the information encoded in the genome," Dr. Leroy Hood, co-founder of the Institute for Systems Biology (ISB) in Seattle, said this summer during a lecture at the Jackson Laboratory in Bar Harbor, Maine.
He and others view biology as dynamic networks that interact with one another and with their environments. Networks change constantly, particularly during development and disease, and a single disruption can reverberate throughout "larger" systems.
Current computational models describe networks of genes and proteins that cooperate in specific tasks, or pathways. But in theory the behavior of an entire cell, groups of cells, or even whole organisms could be simulated - if enough data could be collected and analyzed.
In a study published this past spring, Dr. Hood's laboratory identified gene and protein networks that are disrupted in cells and in tissues affected by prostate cancer. Some proteins in these networks may be secreted into the blood, and the researchers are studying how the secreted proteins, the perturbed networks, and the clinical disease are related.
"The key point of this research is that you need to understand the whole system," says Dr. Biaoyang Lin of ISB, who co-led the study in the April 15 Cancer Research. The field will need new technologies and methodologies, he adds, noting that changes in proteins cannot yet be surveyed in a comprehensive manner.
The field also needs efficient ways to integrate biological information from different sources, such as imaging or microarray studies, in order to build the models.
"These models are where the real power of a systems, or integrative, approach will be utilized," says Dr. Daniel Gallahan, program director of the Integrative Cancer Biology Program at NCI. "The models will begin to transform how we conduct research as they become more sophisticated and accurate."
Though they are approximations of reality, models can generate hypotheses to be tested experimentally, and the results of experiments can, in turn, improve models.
"Modeling is a powerful tool for designing, testing, and refining hypotheses about cancer, and it goes hand in hand with experimentation," says Dr. Thomas Deisboeck of the Complex Biosystems Modeling Laboratory at Massachusetts General Hospital.
Dr. Deisboeck leads an international project to develop a virtual tumor that could simulate the ripple effects of a single mutation on multiple cells and tissues. "We view cancer as a complex adaptive biosystem, and we're using various modeling techniques to study its dynamics at multiple scales in space and time," he says.
The project, which also is developing Web-based infrastructure for the cancer-modeling community, is one of nine supported by NCI's Integrative Cancer Biology Program. Each project will generate computational models that simulate a different aspect of the cancer process, such as communication among cancer cells.
"Some believe that integrative approaches are really the only way to understand biology because biology does not occur in a vacuum," notes Dr. Gallahan. "There is always an environment, whether it's a cell or a tissue or something else."
The interconnectedness of biology is an old idea; what distinguishes systems biology from its predecessors, such as physiology, is the focus on modeling and high-throughput technologies.
A recent editorial in Molecular Systems Biology endorses the new term systems biology - even if physiology could have been revived: "After all, a new generation of scientists enabled all the progress, and a new term always creates hope and stimulates visions."
By Edward R. Winstead
|
 |
 |
Prophylactic Antibiotic Use Effective in Patients Undergoing Chemo
Two new double-blind, randomized clinical trials suggest that the prophylactic use of the antibacterial agent levofloxacin can reduce the risk of fever, bacterial infections, and hospitalizations in patients undergoing chemotherapy who are likely to develop neutropenia, a dangerously low white blood cell count. Both studies were published in the September 8 New England Journal of Medicine (NEJM).
The studies - one conducted in Italy, the other in the United Kingdom - had slightly different designs. The Italian study randomized 760 patients about to undergo chemotherapy and who were at risk for prolonged neutropenia (7 days or more) to receive levofloxacin or placebo until the neutropenia resolved. Patients in the treatment group were less likely to develop a fever, which was the study's primary endpoint because it is indicative of an infection, than those in the placebo group (65 percent vs. 85 percent). Treatment-group patients were also less likely to have documented cases of bacterial infections than the placebo group. Antibacterial prophylaxis did not improve survival compared with placebo.
The second study involved 1,565 patients scheduled to undergo cyclic chemotherapy who were randomized to a placebo or levofloxacin for 7 days when the risk of neutropenia was highest. In the first chemotherapy cycle, 3.5 percent of patients in the treatment group developed a fever, compared with 7.9 percent of those on placebo. Patients on levofloxacin also had lower hospitalization rates, 15.7 percent vs. 21.6 percent. Again, no difference in survival was documented.
The prophylactic use of antibacterial agents has been controversial, especially because of concerns about increasing the spread of drug-resistant bacteria. Neither study was designed to specifically address the issue of resistance, although one study did show an increase in drug-resistant bacteria in those treated with levofloxacin.
"One way to maintain the benefits described in the two current studies but minimize the risk [of bacterial drug resistance] is to restrict its use to those at highest risk," suggested Dr. Lindsey R. Baden, an NEJM deputy editor, in an accompanying editorial. "Efforts to improve risk stratification will be critical to minimize unnecessary use of antimicrobial agents and simultaneously preserve the benefits described in the two studies."
Survivors of Testicular Cancer at Long-Term Risk for Second Cancers
Men who survive testicular cancer face an increased risk of developing a second cancer for at least 35 years after treatment, and young patients may face increasing levels of risk as they get older, according to the largest study to date of testicular cancer survivors.
Ten-year survivors who had been diagnosed with testicular cancer at age 35 had twice the risk of developing a second cancer as someone in the general population - and this risk remained elevated for 35 years. Patients diagnosed at younger ages faced higher risks than those diagnosed at older ages.
Testicular cancer primarily affects young men and is largely curable, but second cancers arising from the late side effects of treatment - especially radiotherapy - are a leading cause of death among survivors. Cancers of the bladder, colon, lung, pancreas, and stomach accounted for 60 percent of those reported, according to findings in the September 21 Journal of the National Cancer Institute (JNCI).
Dr. Lois B. Travis, of NCI, and her colleagues examined data from more than 40,000 survivors from tumor registries in Europe and North America, and identified a total of 2,285 second solid cancers. They found for the first time significant excesses of malignant mesothelioma and esophageal cancer, likely reflecting the past use of chest radiotherapy.
The researchers note that despite the risks of second cancers, it is clear that "the remarkable gains in survival provided by treatments for testicular cancer far outweigh the risk of this serious late effect." In the July 20 JNCI, Dr. Travis and her colleagues reported that the long-term risk of testicular cancer patients developing a second cancer in the opposite (contralateral) testicle is very low.
Letrozole Study Update Confirms Drug's Benefits
After preliminary data showed improved disease-free survival among women with estrogen receptor-positive breast cancer who received letrozole following tamoxifen, results from the MA.17 clinical trial were announced in 2003, a year ahead of schedule, and participants in the placebo group were unblinded and offered the study drug instead. The study team recently released a more detailed analysis of the drug's efficacy and toxicity up to the time of unblinding. Their findings are published in the September 7 JNCI.
The updated data from 5,170 patients show that after 4 years of follow-up, 94.4 percent of women who received letrozole survived without disease recurrence, compared with 89.8 percent of those who received the placebo. In general, women who received letrozole and women who took the placebo saw no difference in overall survival, though the drug did seem to improve overall survival among a subset of women who had positive axillary lymph nodes, as well as those who had taken tamoxifen for more than 5 years. Toxic side effects of concern included those related to bone metabolism and cardiovascular disease, but bone fractures and cardiovascular events occurred equally between the two study groups.
"[Our preliminary findings] have changed clinical practice, but because the median follow-up was short the question of duration of therapy remains unanswered for the time being," the authors conclude, indicating that analysis of data from patients who switched to letrozole after the 2003 unblinding may provide the answer.
Anti-Seizure Drug Reduces Breast Cancer Hot Flashes
Hot flashes are one of the most common symptoms in women receiving treatment for breast cancer, especially hormone treatments such as tamoxifen. In a prospective, randomized, double-blind, placebo-controlled study of 420 breast cancer patients experiencing hot flashes, the anticonvulsant drug gabapentin reduced these symptoms by about half in some women. The multicenter study was based at the University of Rochester Medical Center in New York and published in the September 3 Lancet.
Nonhormonal treatments for hot flashes became a priority in 2002 when early results from the National Institutes of Health Women's Health Initiative showed adverse effects related to hormone replacement therapy, including increased breast cancer risk. Drugs such as the antidepressant venlafaxine and the antihypertensive clonidine are currently used to mitigate hot flashes. Lead author Dr. Kishan J. Pandya said in an interview that gabapentin now provides another good alternative, especially for patients already taking antihypertensives. Gabapentin is FDA-approved to treat epilepsy, and is also used for neuropathic pain.
The women in the study took a placebo, 300 mg of gabapentin, or 900 mg of gabapentin each day for 8 weeks and kept a diary to describe their hot flashes. The 900 mg dose produced significantly better results than the 300 mg dose after 8 weeks, reducing the frequency of hot flashes by 44 vs. 30 percent, and the severity by 46 vs. 31 percent. Other menopause symptoms among the three groups were not significantly changed, except for suppressed appetite and decreased pain in the 900 mg group of women.
Erlotinib Approval Recommended for Pancreatic Cancer
A Food and Drug Administration (FDA) advisory panel last week recommended that the agency approve the targeted agent erlotinib (Tarceva) for use in combination with gemcitabine (Gemzar) to treat patients with advanced pancreatic cancer. The approval was based on the results of a clinical trial announced earlier this year in which the combination offered a small, but statistically significant, improvement in survival compared with gemcitabine plus placebo.
Members of the panel, the Oncologic Drugs Advisory Committee, struggled with whether a single trial demonstrating a nearly 13-day improvement in median survival - which could be accompanied by severe bouts of diarrhea and rash, as well as lower rates of life-threatening events such as stroke - was enough to justify an approval recommendation.
Despite the lack of options for these patients, several committee members, including the chair, Dr. Silvano Martino from the USC Keck School of Medicine, and Dr. Bruce Cheson from the Lombardi Comprehensive Cancer Center at Georgetown University, were not convinced that the data were compelling enough. But most committee members, while acknowledging the minimal benefit provided by adding erlotinib to gemcitabine, felt it represented a first step in the right direction.
"This was a positive study in a tough disease," said committee member Dr. Maha Hussain from the University of Michigan. "I don't think we ought to be the judge on what patients would choose."
The median survival and adverse events data could be misleading, added Dr. Mace Rothenberg, from Vanderbilt-Ingram Cancer Center. Diarrhea and rash could be controlled by decreasing the dose of the drug, he noted, while data on average survival showed that many patients in the erlotinib arm lived 5 weeks or so longer than those in the placebo arm.
|
 |
 |

Chemotherapy for Advanced or Recurrent Endometrial Cancer
Name of the Trial
Phase III Randomized Study of Doxorubicin, Cisplatin, Paclitaxel, and Filgrastim (G-CSF) versus Carboplatin and Paclitaxel in Patients with Stage III or IV or Recurrent Endometrial Cancer (GOG-0209). See the protocol summary at http://cancer.gov/clinicaltrials/GOG-0209.
 Principal Investigator
Principal Investigator
Dr. David Scott Miller, Gynecologic Oncology Group
Why Is This Trial Important?
Endometrial cancer is cancer that arises from the lining of the uterus (the endometrium). It is the most common form of gynecologic cancer. Although endometrial cancer is curable in its early stages, advanced disease is often fatal.
Currently, there is no standard treatment for endometrial cancer that has spread beyond the uterus or recurred. Doctors treating patients with such cancers usually rely on one of two chemotherapy combinations: treatment with the drugs doxorubicin, cisplatin, and paclitaxel plus the biologic agent filgrastim (TAP) or with a combination of carboplatin and paclitaxel (TC).
In this trial, researchers will compare these two chemotherapy combinations in women with advanced or recurrent endometrial cancer to determine which combination will help patients survive longer. Additionally, the researchers will assess the patients' quality of life and examine the side effects of each combination.
"This is the most recent in a series of trials evaluating new drug treatments for endometrial cancer," said Dr. Miller. "In our previous studies, TAP emerged as the leading treatment choice. Many oncologists, however, use the TC combination, which is also used to treat ovarian cancer.
"This study is designed to set the standard of care for women with advanced endometrial cancer by comparing these two leading chemotherapy combinations," Dr. Miller said.
Who Can Join This Trial?
Researchers seek to enroll 900 patients aged 18 or over with confirmed stage III or stage IV or recurrent endometrial cancer who have not previously undergone chemotherapy. See the list of eligibility criteria at http://cancer.gov/clinicaltrials/GOG-0209.
Where Is This Trial Taking Place?
Multiple study sites in the United States are recruiting patients for this trial. See the list of study sites at http://cancer.gov/clinicaltrials/GOG-0209.
Contact Information
See the list of study contacts at http://cancer.gov/clinicaltrials/GOG-0209 or call the NCI's Cancer Information Service at 1-800-4-CANCER (1-800-422-6237). The call is toll free and completely confidential.
An archive of "Featured Clinical Trial" columns is available at http://cancer.gov/clinicaltrials/ft-all-featured-trials.
|
 |
 |
 |
 |
Director's Intramural Award Program Seeks Applications
The NCI Director's Intramural Innovation Award Program is designed to support the development of highly innovative approaches and technology aimed at significant cancer-related problems. Novel proposals with the potential for high impact are sought from NCI's CCR and DCEG. One-time awards for FY06 are offered at two levels: PI Awards with an upper limit of $50,000 are targeted to tenure-track or recently tenured PIs; Career Development Awards with an upper limit of $10,000 are targeted to postdoctoral fellows, staff scientists, staff clinicians, and senior scientists.
Letters of Intent are due by September 30, 2005. Applications are due by October 21, 2005. Please direct questions, letters of intent, and applications to: Janelle Cortner at jc328z@nih.gov or 301-846-5712 (CCR staff) or Marianne Henderson at mk149c@nih.gov or 301-496-8672 (DCEG staff).
|
 |
|
|
Warning Issued on Herceptin Cardiotoxicity
A letter has been sent to oncologists and others caring for cancer patients about new data on cardiotoxicity related to the use of trastuzumab (Herceptin) by the drug's manufacturer, Genentech. The data come from a phase III clinical trial being conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP) testing trastuzumab in women with operable, HER2-expressing breast cancer. The study, NSABP B-31, is evaluating the addition of trastuzumab to standard adjuvant chemotherapy compared with adjuvant chemotherapy alone.
The data show a significant increase in cardiotoxicity in 18.6 percent of patients in the trastuzumab arm of the study, including an asymptomatic decrease in left ventricular ejection fraction, or LVEF (a measure of how well the heart is pumping), and in symptomatic cardiac dysfunction or other cardiac toxicity. In addition, there was a statistically significant increase in advanced cases of heart failure (New York Heart Association Class III and IV) compared with those in the control arm (4.1 percent vs. 0.8 percent).
Final analysis of the study's cardiac safety data - as well as data from a similar trial being conducted by the North Central Cancer Treatment Group (NCCTG), N9831 - is ongoing, the letter explains. "Risk factors for cardiac dysfunction will be analyzed with data from both the NSABP B-31 and NCCTG N9831 trials, when available," the letter continues. "A preliminary exploratory analysis performed by NSABP investigators suggests that age and LVEF following AC chemotherapy may identify patients at greatest risk for symptomatic cardiac dysfunction."
For more information, go to the FDA's MedWatch site.
NCI Hosts Translational Immunology Conference
NCI's Center for Cancer Research and the Center of Excellence in Immunology will host a 2-day conference focusing on novel immunotherapy strategies for the prevention or treatment of a range of human cancers. The conference will be held September 22-23 in Masur Auditorium, Building 10, on the NIH campus in Bethesda, Md. This presentation will be webcast at http://videocast.nih.gov. For more information on the conference, visit http://web.ncifcrf.gov/events/tirc.
 |
 |
CCR Grand Rounds |
 |
|
September 27: Dr. James B. Mitchell, Chief, Radiation Biology Branch; CCR, NCI; "Chasing Free Radicals: Use of Nitroxide Antioxidants in Cancer Treatment and Prevention"
October 4: Dr. Pamela Hinds, Director of Nursing Research, St. Jude Children's Research Hospital; "Fatigue in Adolescents with Cancer"
CCR Grand Rounds are held 8:30 to 9:30 a.m. at the NIH campus in Bethesda, Md., in the Clinical Center's Lipsett Amphitheater.
|
|
IMAT PI Meeting Highlights Strengths of NCI Technology Development
The sixth annual principal investigator's (PI) meeting of NCI's Innovative Molecular Analysis Technologies (IMAT) program was held September 7-9 in Washington, D.C. Dr. Erkki Ruoslahti presented the keynote address, and representatives from FDA, the venture capital community, and large and small businesses provided their perspectives to more than 100 PIs and NCI program managers who attended the meeting. Investigators shared research progress and discussed potential collaborations with a broad range of scientific, multidisciplinary, and cross-sector experts. The next receipt date for IMAT applications is October 18, 2005. See http://otir.cancer.gov for more information on the meeting and the IMAT program.
Science Writers' Seminar to Focus on Behavioral Aspects of Cancer
The next NCI Science Writers' Seminar will take place on October 5 at Fox Chase Cancer Center in Philadelphia. Experts from NCI and Fox Chase will discuss the different ways in which physicians and patients react to, communicate, and make decisions about a diagnosis of cancer. Dr. Michael Stefanek of NCI and Drs. Mary Daly, Suzanne Miller, and Neal Meropol of Fox Chase will present information on how basic decision making can be integrated into cancer control activities, how patients and physicians think about clinical trials, and how different types of people process health information.
The event will begin at 11:00 a.m. and will be webcast simultaneously at http://videocast.nih.gov. The webcast will be archived for later viewing. For additional information, contact the NCI Press Office at (301) 496-6641 or at ncipressofficers@mail.nih.gov.
Weinberg to Lecture on Tumor Formation
On September 28 at 9:00 a.m., in the Lipsett Amphitheater in the NIH Clinical Center, Dr. Robert A. Weinberg will discuss "Mechanisms Leading to the Formation of Human Malignancies." The lecture is part of the NCI/NIH Stars in Nutrition & Cancer Seminar Series.
A professor of biology at Massachusetts Institute of Technology and a founding member of the Whitehead Institute, Dr. Weinberg is a pioneer in cancer research, most widely known for his discoveries of the first human oncogene and the first tumor-suppressor gene.
|
|
 |
 |

Where's Sputnik?

 We Americans have a tradition of rising to a challenge. We fought for and won our independence. Later, when the Civil War tore the country apart, we bound up our wounds and created a stronger nation. And when the Soviet Union launched Sputnik, the first man-made Earth satellite, we responded by putting astronauts on the moon in little more than a decade.
We Americans have a tradition of rising to a challenge. We fought for and won our independence. Later, when the Civil War tore the country apart, we bound up our wounds and created a stronger nation. And when the Soviet Union launched Sputnik, the first man-made Earth satellite, we responded by putting astronauts on the moon in little more than a decade.
But when it comes to cancer, there is no Sputnik, no single shocking event that galvanizes our response. Cancer is so common - half of all men and a third of women will be diagnosed - that our society has come to think of it as almost normal. Fortunately, "normal" can change over time. When I was a child, the 4-minute mile was considered a nearly impossible achievement. Yet once Roger Bannister, a young medical student, broke through that psychological barrier, 16 other runners beat the 4-minute "limit" within a year.
Under the leadership of Dr. Andrew von Eschenbach, NCI has outlined a program to eliminate cancer as a cause of death and suffering by 2015. While a partnership of government, academic, and private industry researchers will lead that effort, there's also an important role for those of us who are patients.
 |
 |
Featured Meetings and Events |
 |
|
Like millions of Americans, I've experienced the devastating effects of cancer firsthand. Before my own diagnosis, I'd already lost 10 relatives, including my father and mother-in-law, to the disease. After two decades of focusing on all cancers, I founded the Prostate Cancer Foundation in 1993. Seven years ago, it was my privilege to help lead hundreds of thousands of patients and advocates on the National Mall and in cities across the country demanding "No More Cancer!" Since then, Congress has nearly doubled America's cancer research budget. In 2003, we launched FasterCures - a Washington, D.C.-based center of the Milken Institute - to accelerate the pace of discovery for all deadly diseases. The FasterCures PHD Program - Patients Helping Doctors - highlights the crucial role individual patients can play in the search for cures by releasing their medical records, donating tissue samples, and participating in clinical trials. (For more information, go to http://www.fastercures.org.)
Other keys to finding cures faster include full implementation of the National Health Information Network and expansion of the cancer Biomedical Informatics Grid initiative to link cancer researchers nationwide. Technology can also bridge the gap between research and care by putting patients in the center of the process and giving them electronic access to and control over their clinical data.
More than 30 years after the National Cancer Act, we've made some important progress. Cancer patients like Bob Dole and John Kerry have run for President and seven-time Tour de France winner Lance Armstrong has shown that even metastasized cancer need not be a barrier to great achievement. Yet as a society, we can do more - much more. For the first time in history, we hold the potential of eliminating cancer's burden. It will take a monumental effort, but the rewards will benefit all future generations.
Michael Milken
Chairman
FasterCures/The Center for Accelerating Medical Solutions
|
 |
|