 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Syphilis Testing Algorithms Using Treponemal Tests for Initial Screening --- Four Laboratories, New York City, 2005--2006In the United States, testing for syphilis traditionally has consisted of initial screening with an inexpensive nontreponemal test, then retesting reactive specimens with a more specific, and more expensive, treponemal test. When both test results are reactive, they indicate present or past infection. However, for economic reasons, some high-volume clinical laboratories have begun using automated treponemal tests, such as automated enzyme immunoassays (EIAs) or immunochemoluminescence tests, and have reversed the testing sequence: first screening with a treponemal test and then retesting reactive results with a nontreponemal test. This approach has introduced complexities in test interpretation that did not exist with the traditional sequence. Specifically, screening with a treponemal test sometimes identifies persons who are reactive to the treponemal test but nonreactive to the nontreponemal test. No formal recommendations exist regarding how such results derived from this new testing sequence should be interpreted, or how patients with such results should be managed. To begin an assessment of how clinical laboratories are addressing this concern, CDC reviewed the testing algorithms used and the test interpretations provided in four laboratories in New York City. Substantial variation was found in the testing strategies used, which might lead to confusion about appropriate patient management. A total of 3,664 (3%) of 116,822 specimens had test results (i.e., reactive treponemal test result and nonreactive nontreponemal test result) that would not have been identified by the traditional testing algorithms, which end testing if the nontreponemal test result is nonreactive. If they have not been previously treated, patients with reactive results from treponemal tests and nonreactive results from nontreponemal tests should be treated for late latent syphilis. Four New York City laboratories that routinely conduct syphilis testing using EIA treponemal screening tests were able to provide their testing algorithms, test volume, and test results for a convenience sample of specimens. Each laboratory used a slightly different testing algorithm and tested approximately 26,000--130,000 specimens for syphilis per year. CDC reviewed test results from a convenience sample of 116,822 specimens tested at these four laboratories during October 1, 2005--December 1, 2006. In all four laboratories, no further testing was done on specimens that were nonreactive with the treponemal screening EIA. In all four laboratories, specimens considered reactive by EIA test were next tested with a rapid plasma reagin (RPR) test. However, the approach to follow-up testing then differed. At two laboratories, specimens that were reactive with EIA and nonreactive with RPR were retested using a different treponemal test: Treponema pallidum particle agglutination (TP-PA) or fluorescent treponemal antibody (FTA-ABS). At a third laboratory, specimens that were reactive to both the EIA test and the RPR test were retested using a different treponemal test (i.e., FTA-ABS or TP-PA). At the fourth laboratory, no further testing was done after the EIA and RPR tests. Of the 116,822 specimens included in the convenience sample, 6,587 (6%) were initially reactive to the EIA test (Figure). When 6,548 of the EIA-reactive specimens were tested with an RPR test, 2,884 (44%) were reactive and 3,664 (56%) were nonreactive to the RPR test. Further testing with FTA-ABS or TP-PA tests on 2,512 of the specimens reactive to the EIA test but nonreactive to the RPR test found 2,079 (83%) specimens reactive to the second treponemal tests (i.e., FTA-ABS or TP-PA). In addition, the one laboratory that performed TP-PA testing on specimens that were reactive to both the EIA and RPR tests found 78 of 80 (98%) specimens were reactive to the TP-PA test. One laboratory provided limited interpretation of the various permutations of syphilis test results. The other three laboratories gave providers an objective summary of the test results (e.g., EIA reactive, RPR reactive, or EIA reactive and RPR nonreactive) with no interpretation. No additional information was available from the four laboratories regarding patient treatment. Reported by: T Peterman, MD, J Schillinger, MD , S Blank, MD, S Berman, MD, R Ballard, PhD, D Cox, PhD, R Johnson, MD, S Hariri, PhD, N Selvam, PhD, Div of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, CDC. Editorial Note:In the four New York City laboratories studied, reversing the traditional order of screening and confirmatory tests for syphilis resulted in 3,664 (3%) of 116,822 specimens with test results (i.e., reactive treponemal test result and nonreactive nontreponemal test result) that would not have been identified by the traditional testing algorithm. The importance of these test results is unclear because no specific prognostic information exists to guide patient evaluation and treatment. Treponemal tests detect antibodies specific to T. pallidum. In addition to T. pallidum pallidum, which causes syphilis, other treponemal subspecies (e.g., pertenue, which causes yaws, and carateum, which causes pinta) also can produce reactive results to treponemal tests, but these subspecies are rare in the United States (1). A reactive treponemal test result indicates that treponemal infection has occurred at some point in the past but cannot distinguish between treated and untreated infections. As such, treponemal tests, such as the T. pallidum EIA test, TP-PA test, and FTA-ABS test, can produce reactive results for life, even after adequate treatment for syphilis. Nontreponemal tests, such as the RPR test and venereal disease research laboratory (VDRL) test, detect antibodies to cardiolipin and are not specific for treponemal infection. Nontreponemal tests are more likely than treponemal tests to produce nonreactive results after treatment; therefore, reactive results from nontreponemal tests are more reliable indicators of untreated infection. Quantitative nontreponemal tests also are used to monitor responses to treatment or to indicate new infections. False-positive nontreponemal tests occur in 1%--2% of the U.S. population, and have been associated with multiple conditions, including pregnancy, human immunodeficiency virus (HIV) infection, intravenous drug use, tuberculosis, rickettsial infection, spirochetal infection other than syphilis, bacterial endocarditis, and disorders of immunoglobulin production (2,3). Nontreponemal test results might be falsely negative in longstanding latent infection (4). Both treponemal and nontreponemal tests can produce nonreactive results when the infection has been acquired recently; approximately 20% of test results are negative when patients have primary syphilis (4). The four New York City laboratories in this report used various algorithms to evaluate specimens that were reactive to treponemal tests and nonreactive to nontreponemal tests. The different algorithms might lead to confusion in the interpretation of test results and, in turn, in the management and treatment of patients. Test results that would not have been identified by the traditional algorithm were obtained for 3% of the specimens tested for syphilis; thus, such results might be expected to occur several thousand times per year in New York City alone. When results are reactive to both treponemal and RPR tests, persons should be considered to have untreated syphilis unless it is ruled out by treatment history. Persons who were treated in the past are considered to have a new syphilis infection if quantitative testing on an RPR test or another nontreponemal test reveals a four fold or greater increase in titer (health departments maintain registries of past positive tests). When results are reactive to the treponemal test but nonreactive to the RPR test, persons with a history of previous treatment will require no further management. For persons without a history of treatment, a second, different treponemal test should be performed (5). If the second treponemal test is nonreactive, the clinician may decide that no further evaluation or treatment is indicated, or may choose to perform a third treponemal test to help resolve the discrepancy. If the second treponemal test is reactive, clinicians should discuss the possibility of infection and offer treatment to patients who have not been previously treated. Unless history or results of a physical examination suggest a recent infection, such patients are unlikely to be infectious and should be treated for late latent infections, even though they do not meet the surveillance case definition (7). Treatment can prevent severe (i.e., tertiary) complications that can result from untreated syphilis, although the probability of such complications occurring without treatment, while unknown, likely is small (6) Treatment also allows patients to report that they have been treated for syphilis if they ever receive similar results from future treponemal screening tests. Public health departments determine their own priorities for partner notification and other prevention activities; however, because late infections are unlikely to be infectious, they would likely be considered low priority for health department intervention activities. Reversal of the traditional syphilis screening sequence has been driven by economics. For high-volume laboratories, an automated treponemal test can be less expensive than using an RPR test for the initial screening. An important consequence of this reversal is the identification of a combination of reactive and nonreactive test results that would not otherwise have been identified. The clinical interpretation of these results is complicated by the lack of standardized follow-up testing algorithms among the four laboratories, and by the lack of an evidence base with which to judge the merits of each algorithm. Consequently, use of a reversed sequence of syphilis testing might result in overdiagnosis and overtreatment of syphilis in some clinical settings. The recommendations in this report might not be appropriate in countries with different patterns of seroreactivity, systems of health care, and epidemiology of disease. Furthermore, additional analyses are needed that further elucidate the use and total costs of these alternative screening approaches for syphilis, given the anticipated increase in use of treponemal tests for screening in the United States. References
Figure 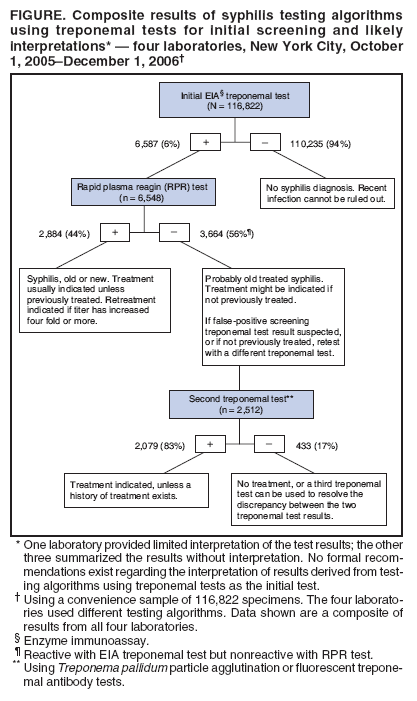 Return to top.
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 8/13/2008 |
|||||||||
|