|
Case study
In December 2005, Lyssenko et al. reported significant associations of the PPARG PP and CAPN10 SNP43/44 GG/TT genotypes with type 2 diabetes (T2D). They suggested that genetic testing for these variants might offer a future approach to identifying individuals at risk of T2D.
Lyssenko V, Almgren P, Anevski D, Orho-Melander M, Sjogren M et al. (2005) Genetic prediction of future type 2 diabetes. PLoS Medicine 2: e345 DOI: 10.1371/journal.pmed.0020345
Lyssenko, et al., analyzed data from a prospective cohort study to investigate the value of genetic factors in predicting type 2 diabetes (T2D). They examined the predictive value of six loci and found that PPARG and CAPN10 genotypes were significantly related to T2D. One of their most striking findings was a 21.2-fold increased risk for T2D in obese carriers of the PPARG PP and CAPN10 SNP43/44 GG/TT genotypes who have elevated fasting plasma glucose (FPG).
1. What are the possible results of a test for PPARG P12A?
a. PP
b. PP or PA/AA
c. PP, PA or AA
d. None of the above
2. What are the possible results of a test for CAPN10 SNP43/44?
a. GG, GA, or AA
b. TT, TC, or CC
c. GG/TT, GA/TC, or AA/CC
d. Any combination of a and b
3. The authors combined PPARG and CAPN10 SNP43/44 in one test.
I. This composite test has 27 possible results
II. The PPARG PP and CAPN10 SNP43/44 GG/TT combination is considered the positive test result.
What is the correct answer?
a. I is correct.
b. II is correct.
c. Both are correct
d. Neither one is correct
4. Calculate the hazard ratios (HR) for the single genetic tests and the combined tests. Which of the following answers is NOT correct?
a. HR for PPARG PP versus other = 1.7
b. HR for CAPN10 SNP43 GG versus other = 1.1
c. HR for CAPN10 SNP43/44 GG/TT versus other = 2.1
d. HR for PPARG PP / CAPN10 SNP43/44 GG/TT versus other = 21.2
In the abstract the authors report a HR of 21.2.
5. How was the HR of 21.2 calculated? (Give your interpretation of this HR. What does it tell you?)
This HR is the ratio of T2D risk in PPARG PP / CAPN10 SNP43/44 GG/TT carriers and
a. T2D risk in the population
b. T2D risk in non-carriers
c. T2D risk in non-carriers with BMI<30 and normal plasma glucose level
d. None of the above
6. What percentage of the study population was in the high-risk subgroup (with HR=21.2)?
7. What does the HR of 21.2 tell you about the value of genetic testing of the aforementioned genotypes for the prediction of T2D?
a. This HR measures the effect of these genotypes on risk of T2D.
b. This HR demonstrates the value of genetic testing for susceptibility to
T2D.
c. This HR does not demonstrate the value of genetic testing for
susceptibility to T2D.
d. Both a and b are correct
Because the HR of the PPARG / CAPN10 SNP43/44 composite test was 3.3 and that of BMI and fasting plasma glucose was 6.0, a test for this genotype does not replace BMI and fasting plasma glucose for the prediction of T2D. We are interested in the extent to which PPARG / CAPN10 SNP43/44 testing can improve the prediction of T2D. For this purpose, we can examine the area under the receiver operating characteristic (ROC) curve for the prediction of T2D based on fasting plasma glucose and BMI, with and without the results of the genetic test. We can do this under one assumption, namely that follow-up time did not differ by genotype.
ROC analysis
The usefulness of composite tests can be evaluated by the area under the receiver operating characteristic (ROC) curve. The ROC curve presents the combinations of sensitivity and specificity for each possible cut-off value of a continuous test result that can be considered to define positive and negative test outcomes. The discriminative accuracy, quantified as the area under the curve (AUC), is determined by the distribution of disease risks in those who will develop the disease and those who will not. The AUC can be interpreted as the probability that the test correctly identifies the diseased subject from a pair in which one is affected and one is unaffected. An AUC of 0.95 means that 95% of the pairs are correctly classified, whereas a test with an AUC is 0.50 is non-discriminative—as accurate as tossing a fair coin. The AUC ranges from 0.5 (total lack of discrimination) to 1.0 (perfect discrimination) and is independent of the prevalence of disease. The magnitude of the AUC indicates whether a test is useful for identifying individuals who are at increased risk of disease (screening; e.g. AUC ~ 0.80) or to diagnose a disease before the onset of symptoms (presymptomatic diagnosis; e.g. AUC >> 0.99).
8. What is the sensitivity and specificity of the combined testing of fasting plasma glucose, BMI and genotype when only the combination of elevated fasting plasma glucose, high BMI and PP/GG/TT is regarded as a positive test result, and all other combinations are considered negative?
a. The sensitivity is 87%
b. The specificity is 99%
c. Both a and b are correct
d. Both a and b are incorrect
The figure shows the ROC curves of the prediction of T2D based on fasting plasma glucose and BMI alone and when genetic testing was added.
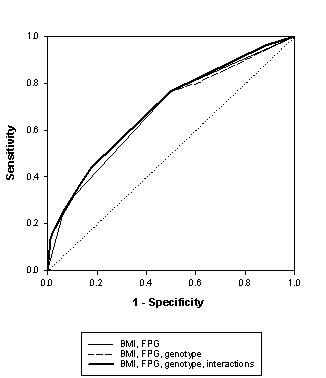
The size of the AUC was 0.68 for the combination of BMI and fasting plasma glucose, 0.68 when the composite genetic test was added, and 0.69 when the prediction model included all possible interaction effects.
9. What is your conclusion about the value of testing PPARG and CAPN10 SNP43/44 genotypes for the prediction of T2D?
a. Testing PPARG and CAPN10 SNP43/44 does not improve the prediction of T2D
b. The discriminative accuracy of T2D prediction based on BMI, fasting plasma glucose and genetic testing is adequate for screening purposes.
c. Both a and b are correct
d. Both a and b are incorrect
10. How do you explain the difference between your conclusion and that of the authors?
a. The authors based their conclusions primarily on an extreme group
comparison.
b. The authors based their conclusions on the HR without considering other
factors necessary to evaluate the usefulness of genetic testing.
c. Both a and b are correct
d. Both a and b are incorrect
| 

