CBER Presentation
Malaria donor deferral policy in France: French experience with malaria antibody screening
FDA Workshop on Testing for Malarial Infections in Blood Donors
July 12, 2006
Olivier GARRAUD
Marie-Hélène ELGHOUZZI
Azzedine ASSAL
Bertrand PELLETIER
Josiane RELAVE
Georges ANDREU
The French situation
- Increasing immigration (7.4% of individuals living in France in 1999); 39.3% of immigrants originate from Africa; Of approx. 136.000 immigrants/year (2003), 90.000 came from Maghreb or Black Africa => approx. 40.000 from Central & West Africa;
- Increasing circulation of individuals: in 2003, France delivered approx. 307.000 visas to travelers from Sub-Saharian Africa (including for transit and short stays)
- Increasing travel and leisure: more than 3 million people living in France travel each year to tropical areas for vacationing, business or visiting relatives
- The 1st cause of fever in returning travelers (from tropical areas) is malaria (42.5% in a French survey in Marseille,BEH-2006)
France is not restricted to te European territory.
Malaria in France
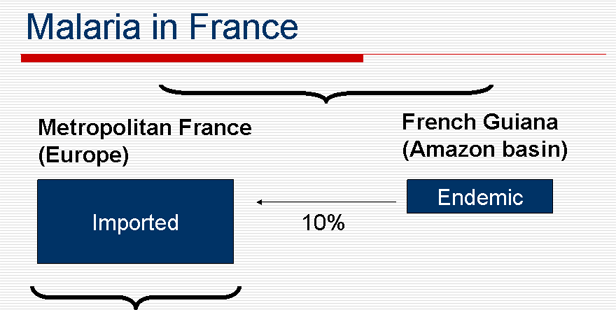
Over 7.000 imported cases p. y. in France (2005): 80% from Africa, 10% from Comores & Mayotte, less than 1% from Guiana
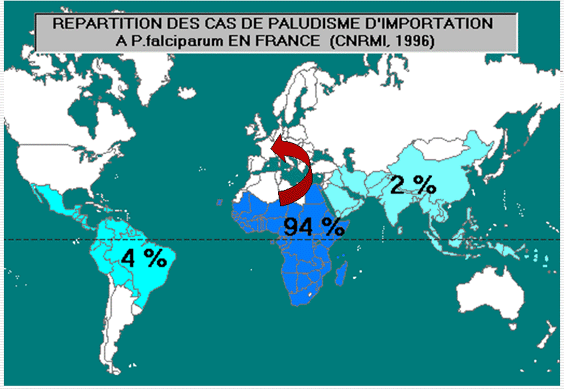
Imported malaria in France
Malaria in France
- France records most imported malaria cases than any other European country ( ≥7,000 cases p. year; Danis, 2005; Legros 2005)
- ≥ 80% imported cases from 13 West & Central African countries + 10 % from Comores and Mayotte (Mayotte is a French District) + a few % from French Guiana.
- ≥ 75% occur in Africans residing in France
- 83.5% P. falciparum, 6.5 % P. ovale, 4.5 % P. vivax (French Guiana) & 1.6 % P. malariae
- 5% cases are "severe"
Transfusion transmitted malarial infection
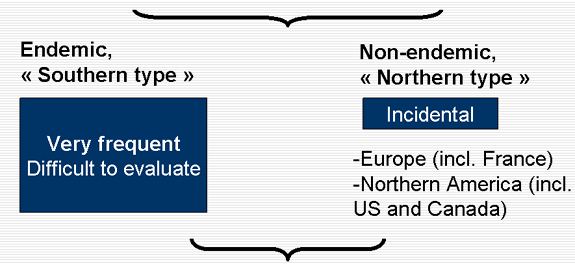
Transfusion transmitted malaria always severe, often lethal
Transfusion-transmitted malaria observed cases over the past 10 years
(after H.W. Reesink, 2004)
| France | 1 | Tunisia | 1 |
| Ireland | 0 | Israel | 1 |
| Italy | 7 | Japan | 1 |
| Spain | 0 | UK | 2 |
| Switzerland | 0 | Canada | 3 |
| Germany | 0 | USA | ≥10? |
History of transfusion-transmitted malaria in France
(after H. Rech, Montpellier, Fr; 2000)
- From 1960 to 1989: approx. 120 reported cases, half of which having occurred between 1975-1989) 1990: 3 cases in the EU, of which 1 in France
- Other cases in France since then:
- 1 in 1993,
- 1 doubtful case in 1998,
- 1 near miss case in 1999,
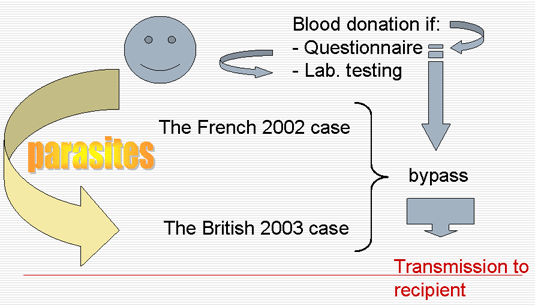
- 1 lethal case in 2002 (Of note: the 2003 British case was very similar to the 2002 French case).
Blood collection in France
- Anonymous, voluntary, non-profit, neither direct nor indirect benefit
- One single governmental organization - unified in 2000 - called "Établissement Français du Sang"
- Subdivided in 14 regions in Metropolitan France and 4 Overseas Blood Centers
- Collection of:
- 2.2 million units of whole blood per year, relatively stable overtime
- 218,000 plasma apheresis procedures, increasing over time
- 169,000 platelet apheresis procedures, increasing overtime
Plasmodiae can be transmitted by labile products (RBC packs; PLT packs)
They do not survive freezing (therapeutic or fractionated plasma)
Transfusion-transmitted malaria prevention in France
- Since the early 90'
- Information and incitation to self-exclusion in case of self history of malaria
- Questionnaire about malaria and exotic travels (1994)
- Anti-malaria antibody testing by indirect single test (recommended: immunofluorescence/IFA) (1995)
Safe enough?
Serology/IFA
- Implemented in 1995
- IFA
- Not standardized between the Biological Qualification platforms
- Could be performed
- Either in the EFS platforms
- Or trusted with University Hospital Platforms
- Results based on a "+" or "-" basis
- Caveats
- IFA difficult to perform and standardize - even within one platform
- Difficult to trace if trusted with outside platforms
- Numerous false positives
- Do not detect Abs to species other than P. falciparum
Of note.
- Lab testing = based on serology
- Malaria and serology do not necessarily fit very well (see the recent TT-malaria case in the US reported by Purdy in 'Transfusion', 2004)
- Parasites and serology do not fit very well in general (Garraud, 'TCB': 2002, 2005)
- Malaria and parasites: '≠' (Garraud, 'Transfusion', in press)
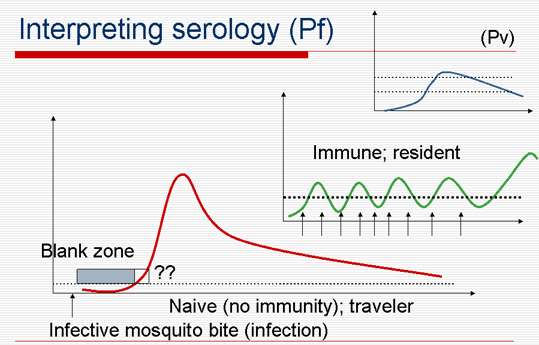
- P. falciparum ±, but P. vivax and P. malariae (and P. ovale) ???
- Silent serological period whatever the test used (variable; depends on individual, prophylaxis, Plasmodium species.)
- => NAT? --- Ag? --- ??? ---
Needs in term of serology to qualify blood => blood safety
- Robustness
- Reliability
- Sensitivity
- Specificity
- Automation is an advantage
| Biological qualification of Blood products is not Clinical Biology:
-different objectives -different requirements |
---------
- Acceptability:
- False positives: (but risk of blood product shortage)
- No false negatives: risk of infectivity
Safety in question:
After the occurrence of the 2002 case =>
Revised measures in France (2005-2006)
- Aug. 2002: all natives (or equivalent) from endemic countries => serology (whatever the time elapsed since return)
- Doubled the # of tests
- Necessitates automation
- Revision of certain measures after the 2002 French case (AFSSaPS/EFS consensus) (2005)
- AFSSaPS is the French National Regulation and Authorisation Authority for Drugs and all products regarding health products (including cosmetics etc.);
- => Blood, Cells, Tissues and grafts
- Implementation of anti-malaria antibody testing by indirect single ELISA test (2005)
- Implementation of the EU directive and novel consensus (EFS) (2006)
- + Brainstorming about procedures of pathogen-inactivation! (safety, implementation and hemovigilance trials: platelets, plasma: Amotosalem, Riboflavin)
Revision of deferral policy
| 3 situations | Policy | Lab. testing |
|---|---|---|
| Reporting of malaria | -Permanent deferral for cellular products -Plasma for fractionation |
-No need of serology |
| -Reported travel in endemic area (listed) - & Less than 3 mo - & Symptomless |
-Temporary deferral for 4 mo for cellular products -Plasma for fractionation |
-Serology to qualify the 1st donation during the period 4 mo -
- 3 y after return (one neg test: OK) |
| -Immigrant from endemic country -or traveler for > 3 consecutive mo - or traveler w. symptoms less than 4 mo after return |
-Temporary deferral for 4 mo for cellular products -Plasma for fractionation -From 4 mo to 3 y: if symptomless & neg serology: OK -OK after 3 y |
-Serology to qualify all donations during the period 4 mo -- 3 y after return |
Revision of lab testing (serology)
- Observational period (2004-2005)
- To obtain information on alternative assays (NAT/PCR, Ag)
- To evaluate both ELISA kits and automated ELISA platforms
- To compare to IFA
- Test period (2005)
- Evaluation of 2 CE-marked ELISA kits (from Diagast, Fr. and from Diamed, CH.)
- Large scale comparison study of ELISA vs IFA
EFS: 4,000 samples from exposed and non-exposed + certain tricky samples from collections - External expertise commissioned
- IPPTS: 10,600 samples from Pf and/or Pv exposed individuals =>IFA vs ELISA
Exploration of false positives and negatives by means of PCR
Selection of an ELISA kit
- CE-marked
- Approved by Regulation authority and experts
- One national market (EFS)
- The Diagast ELISA (4 unidentified rec. Ags) did not pass the evaluation
- The Diamed ELISA (x rec. Ags from Pf and one? from Pv) passed the evaluation
- Diamed ELISA vs IFA
- Same sensitivity
- Limited # of false negatives
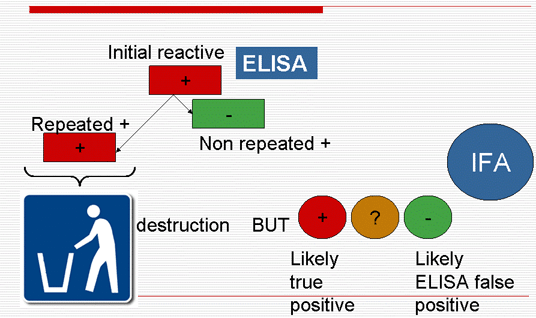
- False ELISA+ different than false IFA+ => ?
- Detects Abs to non-Pf species contrary to IFA, in particular Abs to Pv
- Detects lower Ab titers than IFA (to be confirmed in an even larger series)
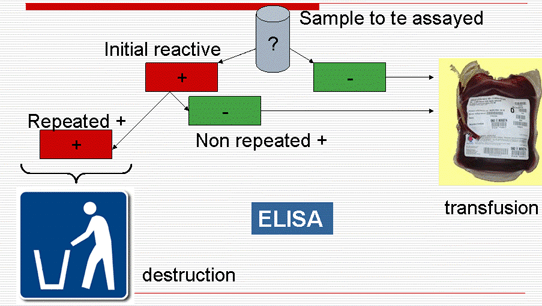
Malaria serology: Decision algorithm
ELISA: qualifying test
Information to blood donor:
IFA (not qualifying test)
Interpreting serology (Pf)
Lab. results
- X serology assays per year in France
- Y positive (x%)
- Doubts subsist on
- Robustness
- Window period
- Detection of Abs to Ags on species other than P. falciparum
------------------------
- Medical interview and lab. testing: Discards approx. 10% total blood donations
Conclusion
- The measures taken in France and in most European countries to prevent Transfusion-Transmitted malarial infection are [almost] sufficient
- The « zero » risk does not exist!
Acknowledgements
- Pr Ermanno CANDOLFI, Strasbourg, France
- Pr. A. Kitchen, UK
- Pr H. Klueter, Germany
- The network of biologists in charge of the qualification of blood donations at EFS
- The Hemovigilance network in France (EFS, hospitals)


