Implementing the Pesticide Registration Improvement Act - Fiscal Year 2007
Resources
PRIA Implementation Reports:Fourth annual report. Report release date: February 29, 2008.
The Consolidated Appropriations Act of 2004 established a new system for registering pesticides, called the Pesticide Registration Improvement Act, or PRIA. The new section 33 of the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), PRIA creates a registration service fee system for applications for specified pesticide registration, amended registration, and associated tolerance actions, which set maximum residue levels for food and feed. Under PRIA, fees are charged for covered applications received on or after March 23, 2004, and for certain pending applications received before that date. The Environmental Protection Agency (EPA) is required to make a determination on the application within the decision times specified. The fee system was authorized until September 30, 2010. Due to the efforts of the PRIA Coalition of industry, trade associations, and public interest groups, PRIA was reauthorized on October 9, 2007 and was effective retroactively to October 1, 2007, the beginning of Fiscal Year 2008. The Pesticide Registration Improvement Renewal Act (PRIA 2) extends authorization of the fee system to September 30, 2012.
Under Section 33(k) of PRIA, EPA is required to publish an annual report describing actions taken under this section during the past fiscal year. The report must include several elements, including a review of the progress made in carrying out the Agency’s obligations under the Act, a description of the staffing and resources associated with the review of and decision-making on applications, and a review of its progress in meeting the reregistration and tolerance reassessment timeline requirements. This fourth annual report covers Fiscal Year 2007 -- October 1, 2006 through September 30, 2007, the last Fiscal Year under the original Pesticide Registration Improvement Act.On this page:
- FY 2007 Enhancements in Application In-Processing
- Financial Overview
- Progress in Meeting Decision Times
- Process Improvements in the Registration Program
- Progress in Meeting Tolerance Reassesment and Reregistration Timelines
- Other Activities
- Appendix A: Decision Review Times for Actions Completed During FY 2007
FY 2007 Enhancements in Application In-Processing
The first annual report, released in March 2005, described steps the Agency undertook to implement PRIA during its first nine months. These included front end processing and screening, waivers, funds management, and communications. In Fiscal Years (FY) 2005 and 2006, these procedures were further refined as described in the second annual report and third annual report respectively. Additional enhancements and preparations for PRIA 2 during FY 2007 are described below.
Front-End Processing and Screening Procedures
To facilitate the implementation of PRIA, the Agency established front-end screening procedures for new pesticide applications in FY 2004. An intra-agency workgroup interpreted the 90 PRIA registration categories to help both applicants and the Agency consistently place each application in the appropriate PRIA category. These PRIA registration categories reflect the types of applications the Agency may receive and for which Congress has established a fee and a time frame. The time frame, or decision review time, is the amount of time the Agency is expected to take to review the application and reach a regulatory decision. The Agency intended to update these interpretations in FY 2007 based on its experience and suggestions provided by stakeholders; however, with the anticipated passage of PRIA 2, the experience and suggestions were incorporated into the 140 PRIA 2 fee categories developed by the PRIA Coalition with the Agency’s technical assistance.
Teams of EPA experts from the three registering divisions (conventional chemical, biopesticide, and antimicrobial pesticides) screen all incoming applications to determine whether they are subject to PRIA and to assign the application to a PRIA category if appropriate. The experts do a cursory screen of the submission for completeness, thus saving both the registrant and the Agency valuable time. Typically within 48-72 hours of receipt of an application, the registrant is sent an invoice requesting payment of the appropriate PRIA registration service fee.
The Agency’s internal tracking system, known as the Pesticide Registration Information System (PRISM) underwent modifications during 2007 to enable the Agency to identify the status of an action and monitor refunds and fee reductions. Additional management reports were developed to identify potentially overdue actions, upcoming actions, and to monitor interim milestones, such as completion of risk assessments, and due dates, more efficiently. These modifications built upon the previous modifications developed for the regulatory process and support data review and risk assessment. The detailed status reports will allow more efficient monitoring of the stages and phases of the regulatory science review process.
The Agency enhanced its existing data management contract for the initial data screen in FY 2004 to reduce study processing time to 10 days, thus ensuring that complete data packages are ready to enter the review process at the beginning of the decision review period if the applicant has correctly formatted the data submission. During FY 2006, the average study processing time for the front end screen was 9.6 days, while in FY 2005 it was 4.6 days. This increase in the average was due in part to delays in processing in May and June 2006 as a result of EPA’s pesticide program move from Crystal Mall 2 to Potomac Yard. Excluding these two months, the average study processing time was about 7 days. In FY 2007, the study processing time was reduced to 4.87 days consistent with the average processing time experienced in FY 2005.
Funds Management and Utilization
Section 33(c) of PRIA established the Pesticide Registration Fund. Congress established this fund in the Treasury of the United States to carry out the provisions of PRIA. All registration service fees received by EPA are deposited in this fund, and expenditures from the fund can cover the costs associated with the review and decision-making for applications for which registration service fees have been paid. In FY 2004, the Agency worked with the Mellon Bank to establish the fund and create billing procedures and to coordinate communications on fee receipts between the bank and the Agency. Communication of the date the fee is received is critical as it triggers the start of the PRIA decision review period, or timeframe. The Agency has been informed of the receipt of a payment within an average of 7.2 days of receipt by the Mellon Bank, and since May 17, 2005, the Agency automatically sends an acknowledgment of payment to those applicants with an e-mail address on file. Effective October 1, 2007, the lockbox was changed from the Mellon Bank to U.S. Bank in St. Louis, Missouri with no break in services.
In July 2005, EPA began notifying applicants when a payment is 45 days overdue for all PRIA fee categories except Fast Track applications (because of the short time frames for these actions). The notification provides the applicant 75 days to forward payment before the application is withdrawn by the Agency. In FY 2006, the Agency sent 94 such letters, resulting in 30 withdrawn applications, 41 payments, 12 fee waivers, and 13 that were subsequently determined not to be PRIA actions. In FY 2007, the Agency sent a fewer number of such letters, 64, resulting in 32 withdrawn applications, 27 payments totaling $890,400, 2 fee waivers, and 3 that were subsequently determined not to be PRIA actions.
For Fast Track applications, the Agency currently informs applicants in an invoice that they have 30 days in which to pay a fee or submit a request for a fee waiver. If neither is received, the application is rejected.
Effective November 1, 2006, fee payments can be made by credit card or wire transfer using the Treasury Department’s pay.gov system. Since that time, payments totaling $1,496,335 have been made through pay.gov for 436 decisions.
When PRIA was implemented, the Agency elected to invoice applicants instead of requiring payment at submission of an application because applicants were unfamiliar with the fee categories. As a result of experience with the fee categories, applicants commented that they wanted the ability to pre-pay the fee or pay it at the time of application. The Agency began an effort to modify its tracking systems to identify such payments. Pre-payment reduces the Agency’s need to invoice applicants and thereby conserves its resources. With the anticipated passage of PRIA 2, the effort was expanded to include the processing changes expected when PRIA 2 was implemented.
Waivers and Fee Reductions
Section 33(b)(7) of PRIA authorizes the Agency to reduce or waive the registration service fee under certain specified situations. The Agency in FY 2004 developed and posted on the internet guidance on how to apply for waivers of the registration service fee. In FY 2007, the Agency reviewed 387 applications and the average number of days to grant a fee waiver was 20 days in the fourth quarter consistent with the activity in FY 2006. The Agency also established formulas for reducing certain registration service fees based on work completed by the Agency prior to the effective date of PRIA. Section 33(b)(8)(C) authorizes EPA to issue discretionary refunds, including instances where the Agency had completed portions of the review of an application before the PRIA effective date. For fees required for pending new active ingredients and for applications where the registrant has offered to pay the registration service fee voluntarily, the Agency applied this refund provision as a credit toward the application registration service fee. The amount the registration service fees were reduced for these instances has decreased each year of PRIA implementation from $3.7 million in FY 2004, to $1.6 million in FY 2005, to $0.8 million in FY2006 and to approximately $3,500 in FY 2007.
Information Management
The Agency, in its efforts to align its information management program with the President’s e-Gov Initiatives, has a number of projects that utilize technology to enhance its ability to serve the public and to implement PRIA.
E-Submission Project - In 2006, EPA’s pesticide program began working toward a more paperless environment by gathering design requirements for the initial phase of an electronic submission system. The long-term goals of the initiative are to reduce data entry, increase transparency, improve processing time, and promote standardization. A pilot system was implemented in March 2007. The system allowed EPA users to upload zip files containing digitized versions of the documents such as studies, labels, and forms submitted by a group of volunteer registrants. The zip files also contained XML (eXtensible Markup Language) files that describe the submitted documents using a prescribed schema adopted from Canada’s Pest Management Regulatory Agency (PMRA) in an effort to promote international harmonization. The e-Submission system unzips the files, parses the XML, performs a variety of validation checks, and stores the “package” and document records in the database. A pilot system interface allows EPA users to review the incoming packages and their data and process them off-line using the Pesticide Registration Information System (PRISM) and other existing systems. The pilot project was completed July 2007. The five registrants participating in the pilot encouraged its implementation. Full implementation is anticipated in May 2008 at which time guidance will be available on the Web.
Documentum - During FY 2007, the Agency invested in Documentum an “Enterprise Document Management System” to improve productivity by providing an electronic mechanism in which all digital assets (electronic files, documents, spreadsheets, etc) are stored, indexed, and retrieved from a central repository, which eliminates the need to store more that one copy of a document in several places. The less time the Agency spends searching for digital assets, the more time it has to devote to completing actions. Time will be saved with these digital assets at the users desktop. An additional benefit to “Electronic document” sharing using Documentum is the ability to share documents simultaneously across the Agency. Eliminating the need to manually search for paper documents improves the overall productivity of the Agency. In many cases, only one copy is available of a paper document. A “proof of concept” was completed and based on the results, the Agency will systematically implement the system in conjunction with the e-Submission effort to reach its goal of a paperless work environment.
Business Objects Upgrade - The Agency advanced its Business Intelligence environment to provide more robust reporting options and more efficiently monitor progress in completing PRIA actions. Upgrades to Business Object XIR2 were completed and training was offered to all employees. The upgrades provided greater flexibility in developing individualized reports. Reports were developed, for instance, to alert employees of actions coming due, to identify actions that had not been closed out or had been improperly closed out of the Agency’s tracking system, and to monitor withdrawals and refunds, PRIA Determinations to not grant, and progress in meeting conventional risk assessment delivery dates.
Communications and Outreach
In 2007, the Agency continued with meetings and other outreach efforts. Agency staff discussed the status of PRIA implementation during the Chemical Producers and Distributors Association Registration Workshop, with State and EPA Regional staff at the Pesticide Regulatory Education Program, and with the Armed Forces Pest Management Board. During the annual meeting of the Consumer Specialty Products Association, EPA and the Natural Resources Defense Council discussed PRIA implementation. EPA provided updates on the status of PRIA actions received and summary statistics during meetings of the Agency’s Federal Advisory Committee, the Pesticide Program Dialogue Committee (PPDC) and meetings with the PRIA Coalition, composed of industry, trade associations, and public interest groups. EPA also has quarterly meetings with the Biopesticide Industry Alliance to discuss PRIA and other common issues and with the United States Department of Agriculture (USDA) IR-4 program, and monthly teleconferences with USDA’s Animal Health Inspection Service and the Food and Drug Administration on Plant Incorporated Protectants. The Antimicrobials Division in coordination with Consumer Specialty Products Association conducted a workshop on May 30 and 31, 2007 for the antimicrobial regulated community on recent policies and procedures and provided guidance on improving applications.
In anticipation of PRIA 2, the Agency, in FY 2007, formulated plans to communicate the impacts of legislative changes to stakeholders. Requirements were developed to modify the PRIA internet site, to develop the PRIA Fee Determination Decision Tree, and to modify tracking and other database systems. Plans were developed for a workshop and other communications materials.
The Agency’s pesticide registering divisions continue to make their processes more transparent by providing additional information to the public on its pesticides internet site such as posting workplans, schedules, and guidance. The Agency maintains a Webpage on this site dedicated to PRIA implementation. This page was modified when PRIA 2 was implemented in October 2007. Through this Website, the public submits questions regarding PRIA implementation. Questions are typically answered within 24 hours. Questions are also addressed by registration Ombudsmen. The Ombudsmen also help applicants with issues related to the registration process and completing application forms.
Financial Overview
During Fiscal Year 2007, the Agency received $13.7 million in new registration service fees and after subtracting $0.62M in refunds (overpayments and withdrawals), net receipts were $13.1 million. A balance of $12.3 million was carried forward from FY 2006. From this total of $25.4 million, the Agency spent approximately $15.1 million, carrying the remaining balance of $10.3 million forward to FY 2008. Consequently, spending increased by 40% in FY 2007 compared with FY 2006, and the end of year remaining balance decreased by 16% in FY 2007 from FY 2006.| For | FY 2004 Expenditures (per thousand) |
FY 2005 Expenditures (per thousand) |
FY 2006 Expenditures (per thousand) |
FY 2007 Expenditures (per thousand) |
|---|---|---|---|---|
| Payroll | $2,535.3 |
$7,898.2 |
$5,819.8 |
$7,111.6 |
| Contracts | $1,591.3 |
$2,228.8 |
$4,013.1 |
$6,979.5 |
| Worker Protection | $430.0 |
$750.1 |
$750.0 |
$750.0 |
| Other Expenses | $455.8 |
$274.3 |
$221.6 |
$302.7 |
| Total | $5,012.5 |
$11,151.4 |
$10,804.5 |
$15,143.8 |
In FY 2007, data review output through contracts continued to increase while the funds spent on payroll costs represented a smaller percentage of funds spent compared with FY 2006. Payroll expenditures increased to $7.1 million in FY 2007 from $5.8 million spent in FY 2006. Expenditures on contracts increased up to approximately $7.0 million in FY 2007, compared with $4.0 million in FY 2006. The end result was nearly an equal balance between payroll and contract expenditures under PRIA in FY 2007 (with payroll at 47% of expenditures in FY 2007 compared with 54% in FY 2006, and contracts were up to 46% in FY 2007 from 37% in FY 2006). The amount spent on worker protection was $0.75 million in contract/grant expenditures. The Agency continued to invest in upgrading its information management systems to track compliance with the PRIA review time frames, to meet reporting requirements, and to prepare for PRIA 2 implementation. Other funds went primarily to pay for FEDERAL REGISTER printing costs associated with PRIA registrations.
Waivers of Registration Service Fees
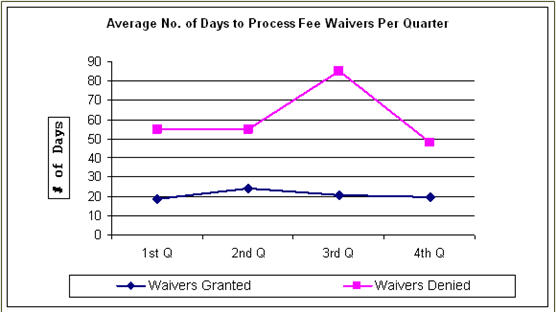
The following is a breakdown of the average number of days EPA took to “Grant” or “Deny” a fee waiver in FY 2007. The breakdown is summarized in the table and illustrated in the graph below. In general, processing times for waivers that were granted remained stable throughout the year, with an increase in the third quarter of FY 2007 when applicants were required to submit complete and updated financial information. On a quarterly basis, processing times for waivers granted decreased from FY 2006 to 20 days in the fourth quarter. The average time to grant a waiver overall in FY 2007 was 21.5 days. The average processing time to deny a waiver was also consistent with FY 2006 except for the third quarter. Only one fee waiver was denied in the third quarter. Due to extenuating circumstances, the applicant was provided additional time in which to submit the required documentation. The time to deny a waiver in the other quarters of FY 2007 was consistent with the average in FY 2006.| Quarter | To Grant | To Deny |
|---|---|---|
| 1st Q | 19 | 55 |
| 2nd Q | 24 | 55 |
| 3rd Q | 21 | 85 |
| 4th Q | 20 | 48 |
PRIA and Pesticide Worker Protection
Under FIFRA Section 33(c)(3)(b), EPA is authorized to use 1/17 of the amount of the Fund (but not more than $1 million and not less than $750,000 for any fiscal year) to enhance current scientific and regulatory activities related to worker protection. The Agency worked closely with worker safety stakeholders through the Agency's Federal Advisory Committee, the Pesticide Program Dialogue Committee (PPDC), to determine which activities to enhance with PRIA funds. Based on the advice of the PPDC, the Agency decided to develop enhancements within focus areas characterized as: Prevention - Safety Training, Response - Poisoning Recognition, Sound Decision Data, and Inform - Risk Management. Within these areas, PRIA funds were used to achieve the following in FY 2007:
- Partnered with AmeriCorps and local farmworker service organizations to give hands-on, interactive pesticide safety training to 75,000 farmworkers, farmworker families, and other members of the agricultural community.
Expanded the scope of a multi-year cooperative agreement with the Association of Farmworker Opportunities Programs (AFOP), which leverages the Agency's funds through agreements with AmeriCorps and local service organizations to provide safety training at 23 sites in 13 states. In addition, a new program was created to provide worker training with local farmworker support organizations in 14 additional states.
- The findings from the pilot study and its report "Evaluation of the Effectiveness of Symbols and Hazard Communication Materials for Migrant Farm Labor" are, where appropriate, being incorporated in the options for change to the agricultural protection regulation.
- Supported the National Agricultural Workers Survey to gather critical demographic data on farm workers and their families by adding questions to the national survey to focus on handler tasks information and farm family exposure potential. Reviewed the preliminary data for use by EPA. The data are informing the development of regulatory change options and are being used to develop training modules to help prevent take-home exposure.
- Supported development of a report through the Department of Labor (DOL) specifically focused on children and youth in agriculture, as well as child labor's exposure to pesticides. The report has been reviewed and is currently awaiting release by DOL.
- Funded the creation and reproduction of EPA pesticide worker safety and Worker Protection Standard (WPS) compliance assistance materials to be distributed through the National Agricultural Compliance Center and EPA Regions, States, and Tribes
10,000 copies of the "Protect Yourself from Pesticides - Pesticide Safety for Agricultural Workers" poster (English and Spanish)
10,000 copies of the "Controlling Heat Stress Made Simple: A Guide to Managing Heat Stress in Agriculture" poster (English and Spanish)
15,000 copies of the "How to Comply with the Worker Protection Standard Regulation for Agricultural Pesticides" manual
72,000 copies of miscellaneous WPS compliance assistance fact sheets.
- Supported the Migrant Clinicians Network (MCN) to develop, test, then evaluate and promote a training model for primary health care providers in practice settings that incorporates key practice skills for the recognition and treatment of pesticide poisonings. In the second year of the 5 year cooperative agreement, the focus was on developing strong partnerships with additional key clinical and health care centers, associations, clinical networks, health professionals, and organizations and agencies dedicated to the migrant population. Building on the first year relationships, more emphasis was placed on the development of resources, dissemination of pesticide-related health information, and the recruitment of 2 additional health care centers to participate in the program.
- Under the Pesticides and National Strategies for Health Care Providers Initiative, an effort to improve the training of health care providers in the recognition, diagnosis, treatment, and prevention of pesticide poisoning among those who work with pesticides, the Pacific Northwest Agricultural Safety and Health Institute did the following in 2007:
- PRIA funds were used to increase the number of states (12) in the National Institute of Occupational Safety and Health Sentinel Event Notification System for Occupational Risk (SENSOR) Program and to expand occupational illness and injury surveillance capacity within state health departments in areas of the country with sizable agricultural worker populations
From 2001-2007, the following twelve states reported occupational pesticide illness and injury cases to the surveillance program: Oregon, Washington, California, New Mexico, Arizona, Texas, Louisiana, Florida, North Carolina, New York, Michigan, and Iowa. During 2007, the program was expanded to Iowa and Michigan.
Built and continued partnerships with a number of organizations, including an active advisory committee that includes representatives from the American College of Occupational and Environmental Medicine (ACOEM), Association of Occupational and Environmental Clinics (AOEC), National Pesticide Medical Monitoring Program (NPMMP), Farmworker Justice, and the University of Washington.
MCN promoted environmental occupational health (EOH) training with an emphasis on pesticide-related issues through 14 training sessions for health care providers (338 attendees), one Web cast clinician training (165 attendees), intensive EOH specific sessions (60 attendees), and distribution of more than 500 pesticide-related resources.MCN updated and maintained its Website with links to partners and access to pesticide-related resources, recording over 20,000 downloads of pesticide-related materials.
MCN’s bimonthly publication included 6 pesticide-related articles; 10,800 newsletters were distributed.
MCN recruited 2 additional health centers to participate in the program, for a total of 4.
Reviewed the curricula in several schools. Defined and developed program-specific curriculum insertion points at Seattle Pacific University nursing program; MEDEX nursing program; University of Washington School of Community Health Family Nurse Practitioner Program; and UW School of Nursing.
Conducted 3 key faculty training sessions at the University of Washington and Heritage University.Continued ongoing development of a teaching materials databank.
Developed screening questions for primary care practitioners to use in their patient intake material.
Developed a database logic model.
Enlisted 2 new student champions from Seattle Pacific University and University of Washington.
Completed two student champion projects, which produced program-specific insertions, reviews of current course content, and an environmental and occupational health history quick reference pocket guide for nurse practitioner positive screening test assessment.
Conducted an "Electronic Pesticide Resource Assessment" to find and & design a way to deliver pesticide teaching modules to educators in the health care fields to fit their teaching styles and meet the demands of individual curricula.
Drafted an emergency department decontamination lab exercise to train students on how to decontaminate after a pesticide exposure.
Continued to update a Webpage for participants that includes resources and materials to be inserted into a university’s curriculum for health care providers.
Progress in Meeting Decision Times
Number of PRIA Actions Completed in FY 2007
The Agency completed 1620 decisions subject to PRIA during the fiscal year, an increase of 273 (21%) over the 1327 reported in the FY 2006 annual report. Among the FY 2007 completed decisions, 308 were antimicrobial decisions, 123 biopesticides and 1189 conventional pesticide decisions. An additional 136 decisions were withdrawn - 35 antimicrobial, 24 biopesticides and 77 conventional. The number completed has consistently increased each year. There was a similar increase (23%) between the decisions completed in FY 2006 and FY 2005.
EPA completed 100% percent of these decisions on or before their due dates according to the Agency’s calculation of its due date. Four missed the statutory due date by one day due to the manner in which the Agency’s tracking system calculates due dates in days using 30 days per month. A three month timeframe becomes 90 days and the Agency’s tracking system will add 90 days to the date the timeframe starts. Since many months contain 31 days, most of the Agency’s target due dates precede the statutory due dates to assure that actions will be completed on time. For decisions that begin their timeframes at the end of January and in February, a three month timeframe contains 89 days since February has 28 days. This issue will not occur in FY 2008, which is a leap year or thereafter. During FY 2008 and as a result of systems modifications to implement PRIA 2, due dates will be calculated in months consistent with PRIA 2 timeframes.
The table below summarizes the number of decisions completed by PRIA category and compares FY 2005, FY 2006, and FY 2007. FY 2005 was the first full fiscal year under PRIA. In reviewing the table, certain factors need to be considered. An application can have more than one decision. The number of decisions depends on the number of product registrations in an application. If a tolerance petition is included in the application, the petition is also assigned a decision number to allow the Agency to track it and assure that it is completed by the PRIA due date for the application. For instance, in FY 2005, one new antimicrobial active ingredient (A2) was registered that required two decisions. Information on the number of active ingredients and uses registered during a year can be obtained in the Office of Pesticide Program’s Annual Reports and should be used in determining whether there are differences in these types of applications between fiscal years. Generally each application categorized as a Fast Track, Non-Fast Track New Product, or Non-Fast Track Amendment contains a single product and is a single decision.
In reviewing the type of decisions that contributed significantly to the increased number of completions, between FY 2005 and FY 2006, an increase in the number of conventional new use (80) and product (105) and antimicrobial non-fast track amendment (42) decisions accounted for the majority of the difference in the number completed. The increase between FY 2006 and FY 2007; however, was primarily due to an increase in the number of conventional new active ingredients (38), new uses (122), and Fast Track new products (70), and antimicrobial new products (35) decisions.
The average decision time for each PRIA category is shown in days and is the number of days it took the Agency to complete a decision once payment was made or a fee waiver was granted. The time frames mandated under PRIA decreased for some categories of decisions in FY 2007. For instance, the time frame for a conventional new food or non-food outdoor use decreased. As an example, the timeframe for an R17 decreased from 22 months to 15 months, and this may be the reason for the increase in conventional new use completions. A decision’s time frame is based on the fiscal year in which the application or decision was received. Even though a fee was paid or a fee waiver was granted in FY 2007, an action received in FY 2006 received a FY 2006 PRIA timeframe. Actions in the same PRIA category completed in FY 2007 may therefore have different mandated timeframes. Consequently, the average decision time or the number of days the Agency took to complete a decision, in the table below can not be directly compared to the PRIA time frames mandated for FY 2007.
Average decision times for conventional non-fast track new products and amendments and non-fast track biochemical new products decreased in FY 2007 from FY 2006. In past years, many new active ingredient and new use applications appeared to have been completed in substantially less time than the decision time frame provided under PRIA. Some of these actions were submitted prior to March 23, 2004, PRIA’s effective date and benefited from work completed before the effective date. As expected, the decision times for these actions, such as R1 to R29, were expected to be greater in future years as more recently received decisions are completed. Average decision times for completing these actions in general were greater in FY 2007 than FY 2006, as predicted. Average decision times for reduced risk new food use active ingredients and new food uses were greater than those of non-reduced risk decisions. The number of reduced risk decisions is sufficiently small that an adequate analysis cannot be conducted to identify a specific reason for this observation. The Agency has observed; however, that reduced risk status is not requested as frequently as in the past. Many new food use decisions are furthermore related to IR-4 minor use tolerance petitions. Risk assessments and regulatory decision making for IR-4 associated applications are conducted with other new use applications submitted for the same active ingredient to conserve the Agency’s resources which reduces the average decision time for these applications.
Among the FY 2007 completions, due dates for 207 (13%) decisions were extended upon mutual agreement of the applicant and the Agency. During FY 2006, fewer (11%) were extended. Extensions generally resulted from missing or deficient data or information. Of all completed conventional decisions, due dates were extended for 7%. The percentage of extensions of antimicrobial (25%) and biopesticide (42%) decisions was higher. Among the 209 decisions with due date extensions, 93 (45%) were non-fast track new product decisions, 14% new active ingredients, 10% new uses, 14% Fast Track New Products, and 17% amendments.
Key to the table
- R - Conventional Pesticides
- A - Antimicrobial Pesticides
- B - Biopesticides
- EUP - Experimental Use Permit
- PIP - Plant-Incorporated Protectants
- SAP - FIFRA Scientific Advisory Panel
- SCLP - Straight Chain Lepidopteran Pheromones
PRIA Category |
Description of Category |
FY 2005 |
FY 2006 |
FY 2007 |
|||
|---|---|---|---|---|---|---|---|
Number |
Average |
Number |
Average |
Number |
Average |
||
| R1 | New Active Ingredient, Food Use | 16 | 365 | 4 | 286 | 17 | 648 |
| R2 | New Active Ingredient, Food Use, Reduced Risk | 8 | 180 | 0 | 10 | 738 | |
| R3 | New Active Ingredient, Food Use, Experimental Use Permit (EUP) submitted simultaneously with application for registration | 0 | 0 | 1 | 634 | ||
| R4 | New Active Ingredient, Food Use, EUP with temporary tolerance, submitted before application for registration | 0 | 0 | 3 | 195 | ||
| R6 | New Active Ingredient, Non-food use, outdoor | 0 | 3 | 423 | 7 | 864 | |
| R7 | New Active Ingredient, Non-food use, outdoor, Reduced Risk | 0 | 0 | 0 | |||
| R8 | New Active Ingredient, Non-food use, outdoor, EUP request submitted simultaneously with application for registration | 0 | 1 | 77 | 2 | 379 | |
| R9 | New Active Ingredient, Non-food use, outdoor, EUP submitted before application for registration | 1 | 354 | 0 | 2 | 205 | |
| R11 | New Active Ingredient, Non-food use, indoor | 0 | 0 | 4 | 832 | ||
| R14 | New Use, Additional food use, indoor Food/Food handling | 2 | 360 | 1 | 489 | 4 | 715 |
| R15 | New Use, First Food Use | 1 | 410 | 0 | 1 | 456 | |
| R17 | New Use, Each Additional New Food Use | 5 | 262 | 47 | 429 | 153 | 646 |
| R18 | New Use, Each Additional New Food Use, Reduced Risk | 11 | 190 | 31 | 617 | 7 | 865 |
| R19 | New Use, Additional New Food Uses, Bundled, 6 or more | 1 | 45 | 18 | 384 | 36 | 691 |
| R20 | New Use, Additional New Food Uses, Bundled, 6 or more, Reduced Risk | 5 | 57 | 2 | 357 | 0 | |
| R23 | New use, Non-food, outdoor | 9 | 281 | 12 | 555 | 23 | 632 |
| R24 | New use, Non-food, outdoor, Reduced Risk | 2 | 115 | 0 | 7 | 538 | |
| R25 | New use, Non-food, outdoor with EUP (no credit toward new use registration) | 2 | 148 | 6 | 112 | 2 | 205 |
| R26 | New Use, Non-food, indoor | 6 | 200 | 7 | 585 | 13 | 507 |
| R28 | Import tolerance, New Active Ingredient or first food use | 0 | 7 | 746 | 2 | 688 | |
| R29 | Import tolerance, Additional new food use | 0 | 2 | 395 | 2 | 597 | |
| R30 | New Product, Me-Too, Fast Track | 222 | 70 | 231 | 68 | 301 | 73 |
| R31 | New Product, Non-Fast Track (includes review of product chemistry, acute toxicity, public health pest efficacy) | 267 | 232 | 342 | 224 | 337 | 183 |
| R32 | New Product, Non-Fast Track, new physical form (excludes selective citations) | 5 | 346 | 16 | 450 | 8 | 356 |
| R33 | New manufacturing-use product, Old Active Ingredient, Selective Citation | 10 | 216 | 20 | 405 | 20 | 472 |
| R34 | Amendment, Non-Fast Track (includes changes to precautionary label statements, source changes to an unregistered source) | 188 | 130 | 136 | 116 | 179 | 111 |
| R35 | Amendment, Non-Fast track (changes to REI, PPE, PHI, rate and number of applications, add aerial application, modify GW/SW advisory statement) | 17 | 130 | 66 | 480 | 45 | 380 |
| R36 | Non-fast track, Isomers | 0 | 2 | 577 | 0 | ||
| R37 | Cancer Reassessment, applicant initiated | 1 | 508 | 3 | 455 | 3 | 785 |
| A38 | New Active Ingredient, Food use, with exemption | 0 | 1 | 350 | 0 | ||
| A41 | New Active Ingredient, Non-food use, outdoor, other uses | 0 | 4 | 288 | 6 | 879 | |
| A42 | New Active Ingredient, Non-food use, indoor, FIFRA sec. 2(mm) uses | 3 | 296 | 12 | 622 | 2 | 644 |
| A44 | New Use, First food use, with exemption | 0 | 0 | 1 | 41 | ||
| A46 | New Food Use, with exemption | 0 | 2 | 392 | 6 | 497 | |
| A47 | New Food use, with tolerance | 0 | 1 | 431 | 0 | ||
| A48 | New use, Non-food, outdoor FIFRA sec. 2(mm) uses | 0 | 1 | 390 | 1 | 261 | |
| A49 | New use, Non-Food, outdoor, other uses | 0 | 0 | 2 | 436 | ||
| A50 | New use, Non-food, indoor FIFRA sec. 2(mm) uses | 2 | 216 | 5 | 282 | 7 | 253 |
| A51 | New use, Non-Food, indoor, other uses | 0 | 3 | 369 | 0 | ||
| A52 | Experimental Use Permit | 1 | 36 | 1 | 270 | 0 | |
| A53 | New Product, Me-too, Fast Track | 79 | 74 | 72 | 83 | 80 | 108 |
| A54 | New Product, Non-Fast Track, FIFRA sec. 2 (mm) uses | 55 | 147 | 48 | 173 | 75 | 178 |
| A55 | New Product, Non-Fast Track, other uses | 5 | 190 | 9 | 243 | 10 | 254 |
| A56 | New Manufacturing use product, old active ingredient, selective citation | 0 | 6 | 481 | 5 | 418 | |
| A57 | Amendments, Non-Fast Track | 64 | 121 | 106 | 107 | 113 | 129 |
| B59 | New Active Ingredient, Food Use, with exemption, Microbial/Biochemical | 6 | 9 | 475 | 9 | 654 | |
| B60 | New Active Ingredient, Non-food use, Microbial/Biochemical | 6 | 293 | 7 | 363 | 6 | 485 |
| B61 | Experimental Use Permit, Food Use with temporary tolerance exemption, Microbial/Biochemical | 0 | 1 | 263 | 5 | 251 | |
| B62 | EUP, Non-food use, Microbial/Biochemical | 0 | 3 | 27 | 1 | 196 | |
| B63 | New Use, First Food Use, with tolerance exemption Microbial/Biochemical, | 2 | 96 | 5 | 490 | 2 | 356 |
| B65 | New Use, Non-Food, Microbial/Biochemical | 1 | 143 | 0 | 2 | 337 | |
| B66 | New Product, Me-Too, Fast Track, Microbial/biochemical | 4 | 74 | 7 | 50 | 14 | 69 |
| B67 | New Product, Non-Fast Track, Microbial/Biochemical | 40 | 196 | 43 | 221 | 35 | 184 |
| B68 | Amendment, Non-Fast Track, Microbial/Biochemical | 14 | 127 | 18 | 122 | 28 | 122 |
| B69 | Straight Chain Lepidopteran Pheromones (SCLP), New Active Ingredient, Food Use or Non-Food Use | 1 | 179 | 4 | 172 | 1 | 235 |
| B70 | SCLP, EUP (New Active Ingredient or New Use) | 3 | 6 | 0 | 0 | ||
| B71 | SCLP, New Product, Me-Too, Fast Track | 8 | 85 | 0 | 5 | 75 | |
| B72 | SCLP, New Product Non-Fast Track | 3 | 189 | 6 | 130 | 6 | 209 |
| B73 | SCLP, Amendment, Non-Fast Track | 11 | 144 | 0 | 0 | ||
| B74 | Plant-Incorporated Protectants (PIP), EUP, Non Food/feed or crop destruct No Scientific Advisory Panel (SAP) meeting | 0 | 0 | 1 | 318 | ||
| B75 | PIP, EUP, with Temporary Tolerance or Exemption, No SAP meeting | 2 | 265 | 2 | 408 | 2 | 268 |
| B80 | PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, No SAP | 1 | 360 | 2 | 498 | 0 | |
| B81 | PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, SAP | 3 | 330 | 0 | 3 | 635 | |
| B86 | PIP, Experimental Use Permit, Food Use, Amendment | 3 | 111 | 3 | 84 | 1 | 147 |
| B88 | PIP, New Product | 2 | 364 | 2 | 349 | 0 | |
| B90 | PIP, Amendment, Non-Fast Track | 7 | 124 | 2 | 179 | ||
| TOTAL | 1098 | 1347 | 1620 | ||||
Note: Appendix A contains a list of all applications subject to PRIA reviewed during FY 2007 (Excel, 276 KB) and includes the decision times for each application. (Microsoft Excel Viewer
is needed to view this file.)
Number of PRIA Applications Pending at the End of FY 2007.
The following table summarizes the pending registration applications (counted as decisions) in each of the PRIA categories. As of September 30, 2007, 1207 applications subject to PRIA were pending in the Agency’s registration queue. At the end of the preceding year, FY 2006, 1256 were pending and are shown for comparison along with FY 2005, the first full year of PRIA. The number pending at the end of a fiscal year does not reflect the number received, since some PRIA categories have multi-year timeframes. Actions are furthermore sporadically received throughout the year, and for decisions with short timeframes, an increase in the number pending at the end of September could possibly reflect additional applications received close to the end of the fiscal year.
Regarding the number of decisions received in FY 2007, more decisions were received than in FY 2006, however due to the increased number of decisions completed, the number pending in FY 2007 was slightly lower than the previous year. The number of decisions received in FY 2007 was 13% greater than that received in FY 2006 with conventional decisions increasing 15%, antimicrobials 8% and biopesticides 10%. The increase in AD actions was due to an increase in the number of new products submitted in FY 2007 (for fee categories, A53, A54, A55 and A56 (152 versus 189). There was a slight decrease in the number of antimicrobial amendments (from 123 in FY 2006 to 112 in FY 2007). Microbial and Biochemical fast track new products decisions increased from 9 in FY 2006 to 22 in FY 2007, and new Plant Incorporated Protectants increased from one in FY 2006 to 10 in FY 2007. A factor in the higher number pending at the end of FY 2006 was an increase in the number of conventional new active ingredients submitted to the Agency (28 in FY 2005 versus 55 received in FY 2006) which are multiyear actions. The number submitted in FY 2007 approximated the number submitted in FY 2006 (49 in FY 2007 and 55 in FY 2006). The increase in the number of conventional decisions received during FY 2007 was due to an increase in the number of new uses (199 in FY 2006 versus 251 in FY 2007), Fast Track new products (257 versus 333), other new products (363 versus 383), and amendments (202 versus 223).
Key to the table
- R - Conventional Pesticides
- A - Antimicrobial Pesticides
- B - Biopesticides
- EUP - Experimental Use Permit
- PIP - Plant-Incorporated Protectants
- SAP - FIFRA Scientific Advisory Panel
- SCLP - Straight Chain Lepidopteran Pheromones
PRIA Category |
Description of Category |
Number of PRIA Decisions Pending at the End of FY 2005 | Number of PRIA Decisions Pending at the End of FY 2006 | Number of PRIA Decisions Pending at the End of FY 2007 |
|---|---|---|---|---|
| R1 | New Active Ingredient, Food Use | 27 | 54 | 48 |
| R2 | New Active Ingredient, Food Use, Reduced Risk | 10 | 22 | 18 |
| R3 | New Active Ingredient, Food Use, Experimental Use Permit (EUP) submitted simultaneously with application for registration | 0 | 1 | 0 |
| R4 | New Active Ingredient, Food Use, EUP with temporary tolerance, submitted before application for registration | 0 | 2 | 0 |
| R5 | New Active Ingredient, Food use submitted after an EUP | 0 | 0 | 17 |
| R6 | New Active Ingredient, Non-food use, outdoor | 10 | 10 | 6 |
| R7 | New Active Ingredient, Non-food use, outdoor, reduced risk | 1 | 0 | 1 |
| R8 | New Active Ingredient, Non-food use, outdoor, EUP request submitted simultaneously with application for registration | 0 | 2 | 0 |
| R9 | New Active Ingredient, Non-food use, outdoor, EUP submitted before application for registration | 0 | 1 | 2 |
| R10 | New Active Ingredient, Non-food use, outdoor, submitted after EUP | 0 | 0 | 3 |
| R11 | New Active Ingredient, Non-food use, indoor | 4 | 6 | 2 |
| R13 | New Use, First food use, indoor food/food handling | 2 | ||
| R14 | New Use, Additional food use, indoor Food/Food handling | 3 | 6 | 5 |
| R15 | New Use, First Food Use | 2 | 9 | 18 |
| R16 | New Use, First Food Use, Reduced Risk | 3 | ||
| R17 | New Use, Each Additional New Food Use | 214 | 278 | 255 |
| R18 | New Use, Each Additional New Food Use, Reduced Risk | 39 | 11 | 11 |
| R19 | New Use, Additional New Food Uses, Bundled, 6 or more | 64 | 81 | 93 |
| R20 | New Use, Additional New Food Uses, Bundled, 6 or more, Reduced Risk | 6 | 4 | 4 |
| R21 | New food use, With EUP and temporary tolerance | 0 | 1 | 2 |
| R23 | New use, Non-food, outdoor | 44 | 43 | 24 |
| R24 | New use, Non-food, outdoor, Reduced Risk | 1 | 7 | 6 |
| R25 | New use, Non-food, outdoor with EUP (no credit toward new use registration) | 3 | 0 | 1 |
| R26 | New Use, Non-food, indoor | 17 | 15 | 4 |
| R28 | Import tolerance, New Active Ingredient or first food use | 12 | 4 | 1 |
| R29 | Import tolerance, Additional new food use | 9 | 10 | 9 |
| R30 | New Product, Me-Too, Fast Track | 45 | 62 | 85 |
| R31 | New Product, Non-Fast Track (includes review of product chemistry, acute toxicity, public health pest efficacy) | 221 | 204 | 189 |
| R32 | New Product, Non Fast Track, new physical form (excludes selective citations) | 17 | 11 | 17 |
| R33 | New manufacturing-use product, Old Active Ingredient, Selective Citation | 25 | 21 | 21 |
| R34 | Amendment, Non-fast Track (includes changes to precautionary label statements, source changes to an unregistered source) | 57 | 68 | 58 |
| R35 | Amendment, Non-fast track (changes to REI, PPE, PHI, rate and number of applications, add aerial application, modify GW/SW advisory statement) | 85 | 55 | 48 |
| R36 | Non-fast track, Isomers | 2 | 0 | 0 |
| R37 | Cancer Reassessment, applicant initiated | 6 | 5 | 6 |
| A38 | New Active Ingredient, Food use, with exemption | 1 | 0 | 0 |
| A41 | New Active Ingredient, Non-food use, outdoor, other uses | 12 | 9 | 5 |
| A42 | New Active Ingredient, Non-food use, indoor, FIFRA sec. 2(mm) uses | 14 | 5 | 5 |
| A44 | New Use, First food use, with exemption | 0 | 3 | 5 |
| A46 | New Food Use, with exemption | 6 | 6 | 4 |
| A47 | New Food use, with tolerance | 1 | 0 | 0 |
| A48 | New use, Non-food, outdoor FIFRA sec. 2(mm) uses | 1 | 0 | 4 |
| A49 | New use, Non-Food, outdoor, other uses | 0 | 3 | 2 |
| A50 | New use, Non-Food, indoor FIFRA sec. 2(mm) uses | 5 | 15 | 10 |
| A51 | New use, Non-Food, indoor, other uses | 3 | 3 | 0 |
| A52 | Experimental Use Permit | 1 | 0 | 0 |
| A53 | New Product, Me-too, Fast Track | 24 | 23 | 22 |
| A54 | New Product, Non-Fast Track, FIFRA sec. 2 (mm) uses | 28 | 36 | 41 |
| A55 | New Product, Non-Fast Track, other uses | 10 | 7 | 4 |
| A56 | New Manufacturing use product, old active ingredient, selective citation | 7 | 6 | 5 |
| A57 | Amendments, Non-Fast Track | 42 | 56 | 50 |
| B59 | New Active Ingredient, Food Use, Microbial/Biochemical, with exemption | 17 | 13 | 11 |
| B60 | New Active Ingredient, Non-food use, Microbial/Biochemical | 11 | 13 | 14 |
| B61 | EUP, Food Use with temporary tolerance exemption, Microbial/Biochemical | 2 | 2 | 2 |
| B62 | EUP, Non-food use, Microbial/Biochemical | 0 | 1 | 0 |
| B63 | New Use, First Food Use, Microbial/Biochemical, with exemption | 10 | 2 | 11 |
| B65 | New Use, Non-Food, Microbial/Biochemical | 0 | 3 | 0 |
| B66 | New Product, Me-Too, Fast Track, Microbial/biochemical | 0 | 2 | 4 |
| B67 | New Product, Non-Fast Track, Microbial/Biochemical | 30 | 29 | 27 |
| B68 | Amendment, Non-Fast Track, Microbial/Biochemical | 8 | 13 | 6 |
| B69 | Straight Chain Lepidopteran Pheromones (SCLP), New Active Ingredient, Food Use or non-Food Use | 0 | 1 | 0 |
| B70 | SCLP, EUP, New Active Ingredient or New Use | 0 | 0 | 0 |
| B71 | SCLP, New Product, Me-Too, Fast Track | 0 | 2 | 0 |
| B72 | SCLP, New Product Non-Fast Track | 3 | 3 | 2 |
| B73 | SCLP, Amendment, Non-Fast Track | 0 | 0 | 0 |
| B75 | Plant-Incorporated Protectants (PIP), EUP, with Temporary Tolerance or Exemption, No Scientific Advisory Panel (SAP) | 2 | 2 | 0 |
| B77 | PIP, EUP, New Active Ingredient, Set Temporary Tolerance or Exemption, SAP | 1 | 0 | 1 |
| B80 | PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, No SAP | 2 | 0 | 1 |
| B81 | PIP, Register New Active Ingredient, Temporary Tolerance/Exemption Exists, SAP | 2 | 3 | 7 |
| B84 | PIP, Register New Active Ingredient, Set Tolerance/Exemption, SAP | 1 | 0 | 2 |
| B86 | PIP, EUP, Food Use, Amendment | 2 | 0 | 3 |
| B88 | PIP, New Product | 5 | 0 | 0 |
| B90 | PIP, Amendment, Non-Fast Track | 3 | 2 | 0 |
Pending Inert Ingredient Reviews at the End of FY 2007
FIFRA section 33(k)(2)(A)(ii) requires EPA to provide the number of inert ingredients pending review by the Agency. In FY 2007, 5 new petitions were received, 5 Final Rules were published, and at the end of the year, 34 petitions were pending. As of December 2007, 26 petitions are in various stages of drafting the final rule (i.e., encoding the Federal Register notices, or Agency comment) and close to completion which will reduce the number pending significantly. All inert petitions are scheduled for review according to date received, with oldest petitions scheduled first on the workplan. The Agency estimates the current average review time as 3-6 months for a polymer exemption petition and 12 -18 months (including data review, science assessment, decision document, and Final Rule) for a new inert petition. All new petitions are screened for deficiencies before being scheduled for review, and EPA works with prospective petitioners to discuss the reliability and adequacy of the data to meet the FQPA safety finding.
Process Improvements in the Registration Program
Section 33(e) of FIFRA directs EPA to identify and evaluate reforms to the pesticide registration process with the goal of reducing decision review times for pesticide registration applications. The Agency has made considerable progress during the fiscal year in improving its operations. We have undertaken a number of steps, both internal and external, to explore, develop, and implement improvements in the registration process.
In identifying process improvements, the Agency will not compromise the scientific quality of its assessments as a means toward reducing decision times. The Agency believes that the best means of gathering recommendations for process improvements is through the Federal Advisory Committee Act (FACA) process.
Pesticide Program Dialogue Committee PRIA Process Improvement Workgroup
The PRIA Process Improvement Workgroup was created in FY 2004 under the auspices of the Agency’s Federal Advisory Committee, the Pesticide Program Dialogue Committee, to evaluate process improvements in the registration program. The workgroup is composed of members from registrant companies, pesticide trade associations, public interest groups, and Agency staff. Meetings are open to the public and are held approximately 2 to 4 times a year. Reports of the April 10 and September 27, 2007, PPDC PRIA Process Improvement Workgroup meetings are posted on the internet when available.
Industry stakeholders identified many areas for improvement in the registration process, including labeling consistency, communication of schedules, use of electronic tools, and difficulties with the application process. Many of the process improvements proposed by the Agency addressed those issues. The Agency continues to work with all stakeholders to evaluate potential improvements to the registration process. During the Workgroup meetings, stakeholders present their priorities for process improvement, and the Agency discusses the status of its improvement projects; previews new tools, procedures and proposed changes in procedures and processes; presents analyses of specific processes; and reports on its successes. Future projects and efforts are identified through a dialogue between the Agency and stakeholders.
Labeling Committee
Both stakeholders and the Agency recognized that labeling issues should be addressed. The Agency formed a cross-program Labeling Committee in FY 2005 to address broad labeling issues and to oversee revisions to the Label Review Manual. A subgroup, the Label Review Manual Team, was formed to revise and continually update the Label Review Manual. During FY 2007, the Team revised an additional nine chapters of the Manual and posted them on the Web. An additional 4 chapters are expected to be posted soon. The entire Label Review Manual is expected to be updated by the end of FY 2008.
The Committee developed a Web site to communicate its activities and to address the public's labeling policy questions forwarded through the Web site’s e-mail address (OPP_labeling_consistency@epa.gov). The Committee received 48 questions during FY 2007 (a total of 126 questions since the site began). Answers to the majority of these questions were posted while some received a direct response. Due to the increase in number of questions and answers, the Agency will again reorganize the Web site. Comments are welcome on changes that would help the users. At the suggestion of stakeholders, the Agency now flags new questions and answers with the word “New” in yellow for 30 days. The date new answers were approved is now placed next to the associated question. To address concerns that responses being posted on the Web site might result in changes to labels already on the market, the Committee placed the following disclaimer at the beginning of the question and answer Web page:
“Answers posted on this web site are intended to provide information to generic questions concerning pesticide labeling. The answers are based on the federal statutes dealing with pesticides, their associated regulations, established policies and guidance. These answers are not intended to create significant new guidance or require any changes to previously accepted labeling unless the answer specifically addresses how problematic labels should be corrected. In addition, the Agency may directly contact registrants on an individual basis about labels of concern. Such changes to EPA accepted labeling will only be required in accordance with standard agency procedures.”
The Committee incorporated labeling recommendations from the Pesticide Program Dialogue Committee's Consumer Label Improvement Workgroup into a Pesticide Registration (PR) Notice and published it for comment. Comments are being reviewed and the Committee expects to publish the final PR Notice in 2008. The Committee also helped draft the policy on “Third-Party Endorsements and Cause Marketing Claims” which was published for public comment. Two issues were posted for comment and finalized during FY 2007: “Contains the Same Active Ingredient” and “Minimum Application Rates. The Committee will continue to consider other issues in FY 2008, and as issue papers are developed, the Committee will place them on the Web site for informal public comment.
Product Chemistry
In FY 2007, analysis of the reasons for PRIA due date extensions continued and again showed that in each of the pesticide registering divisions at least a third of the due date extensions involved product chemistry issues (including inerts issues). To address these issues, all three of the registering divisions developed materials to be included in the Blue Book and on the biopesticides and inert ingredients internet pages to guide applicants in their product chemistry submissions, with a goal of addressing common errors.
The Agency has a number of efforts underway to provide additional guidance and tools to improve product chemistry submissions. For instance, it is exploring developing a tutorial for novices on how to develop a product chemistry submission that would provide a more detailed step-by-step approach to developing a submission. The Agency is also exploring a smart Confidential Statement of Formula (CSF) form for applicants to use. The smart form would, for example, let an applicant know when a required portion of the form had not been completed and would alert applicants if the percent composition column did not add up to 100%. Formation of a subject matter expert group on product chemistry similar to the Labeling Committee is being explored. This group could answer questions on line and keep a list of questions and answers posted on a Web site as well as reference key sites dealing with product chemistry issues.
Process Improvements Implemented within the Pesticide Registration Program
The Agency made a number of process improvements to monitor workload and ensure that PRIA due dates are met. Reports monitor the status of due dates and help managers identify priority actions. In FY 2007, the Agency developed additional status reports to monitor the different stages of the registration process. In providing technical assistance to the PRIA Coalition of stakeholders developing PRIA 2, the Agency analyzed the PRIA categories, its preliminary interpretations, and the category issues identified during the Agency’s experience in implementing PRIA.
The Agency continued to post risk assessments for new conventional pesticides as they were registered during FY 2007 to aid registrants with future submissions. Human health and ecological risk assessments were attached to the new active ingredient fact sheets.
Reviewing labels can be time consuming. Electronic Label Review continues to move forward and reviewing labels is becoming more efficient and accurate. Reviewers use software to compare a label submitted in .PDF format to the previous label to quickly identify where changes have been made. The same software can be used to annotate any required corrections. The marked-up label can be e-mailed to the registrant and then the revised label can be e-mailed to the Agency. All regulatory staff in the Registration, Antimicrobials, and Biopesticides and Pollution Prevention Divisions were trained during 2007. Even though the process and procedures are in place, the use of electronic labels has been slowly implemented. Submissions of electronic labels has risen from about 50/month in early 2007 to about 80/month in late 2007. This, however, is still only a fraction of the total number of labels submitted. Plans for 2008 are to encourage registrants to submit more labels in .PDF format and to provide additional individual staff guidance. In addition, the Agency will begin to categorize the content of submissions so that the number of paper and electronic labels submitted can be counted and progress measured. In FY 2008, the Agency plans on modifying its tracking systems to capture the number of electronic labels submitted and reviewed to generate status reports and to identify issues.
The conventional chemical division reviewed and then revised their notification process to ensure that registrants are notified in writing whether their notifications are acceptable or unacceptable in accordance with Pesticide Registration Notice 98-10. The unacceptable notifications are further reviewed to determine whether they are fast track amendments or PRIA actions. If a PRIA action, it is returned to the applicant for resubmission to the Agency. A team was formed to conduct these reviews with a team leader to review and track the team’s progress. As of FY 2006, notifications are entered into the Agency’s tracking databases. The team leader receives weekly reports of the status of each notification action. The team has significantly reduced the backlog of notifications.
A focus of antimicrobial registration in FY 2007 was to help applicants improve the quality their submissions while increasing internal consistency and efficiency. A workshop was held in FY 2007 at which time the Agency provided additional guidance on the application process and product chemistry submissions. Many of the due date extensions experienced with antimicrobial applications were a result of product chemistry data deficiencies. Meetings were held with antimicrobial stakeholders in an effort to improve applications, and the discussions led to a tentative agreement between the Agency and the stakeholders to hold a workshop on product chemistry early in 2008.
Internally, the Agency conducted intensive training sessions for its antimicrobial regulatory staff on labeling, data requirements, data compensation and other areas relevant in the review of applications; use of the Agency’s information management systems; the statute and regulations; and anticipated changes resulting from PRIA 2. Progress is being made on standardizing the review of both acute toxicity studies and chemistry data through a series of meetings between the pesticide registering divisions to improve consistency among reviewers. Progress continues on establishing new standard operating procedures that will document decisions and promote consistency among science and regulatory reviewers. The Agency continues its consultations with the Center for Disease Control to develop a hierarchical model that will facilitate the Agency’s review process so that antimicrobial products will be available when needed to combat new pathogens. The goal is to develop a Memorandum of Understanding between the two agencies.
The Agency’s success in meeting due dates was a result of its continued monitoring of the status of PRIA decisions and identification of efficiency measures that conserved resources and time. Numerous internal Agency meetings continue to monitor workload and compliance with PRIA due dates. Throughout the pesticide registration program, weekly meetings are held to review the status of pending decisions, due date extensions, and refunds; to identify potential issues and target their resolution; to resolve fee category questions; and to coordinate schedules with science support organizations. Senior managers review justifications and make final decisions to extend or negotiate a PRIA due date and whether or not to issue a “PRIA Determination to Not Grant” a registration. On a bi-monthly basis, progress in meeting PRIA due dates and the short term pending workload are evaluated across all involved organizations and periodically shared with stakeholder groups.
Registration Program Workplans
The multi-year workplan for new conventional chemical actions and new uses under PRIA is updated quarterly. These updates reflect new actions received under PRIA, actions completed, and changes to schedules. For a majority of the new chemical and new use actions listed, the time frame in which the Agency expects to complete its registration decision is shorter than that specified by PRIA. When possible, requests for new uses submitted by USDA’s IR-4 program that are also being requested by registrants are merged into one risk assessment. Additional economies and time-savings were achieved where possible by folding new use assessments into assessments conducted for reregistration and tolerance reassessment.
The FY 2008 workplan for new biopesticide active ingredients is available. The biopesticide workplan is updated at least once a quarter to reflect completed actions and changes to the schedule. Schedules for new antimicrobials and new antimicrobial uses will be posted in the near future.
Science Review Improvements
By taking advantage of geospatial data and analytical techniques, risk assessors can provide better, more accurate, and more relevant information about the potential effects of pesticides in the environment. In FY 2007 the Agency began its transition towards spatially-explicit risk assessments and applying geospatial techniques to its aquatic risk and exposure assessments. With the adoption of the new NHDPlus hydrography network data set, advanced tools and data queries make it possible to analyze the downstream effects of pesticides on aquatic species, including endangered species. In addition, EPA now has the tools and data necessary to analyze effects on drinking water watersheds and is well on its way to be able to assess the effects of non-agriculture uses such as residential and industrial pesticide uses.
A new version of EPA’s aquatic exposure model (PRZM-EXAMS) includes the capability to better simulate irrigation. In previous versions of the model, irrigation was not handled properly. The input shell was improved so that a wider variety of application rates (including different rates on different applications) and methods can easily be modeled. The Agency developed guidance to better describe and characterize both the spatial and temporal variability it expects to find in pesticide levels in water.
To support endangered species assessments, the Agency developed a modification of its terrestrial exposure model to address frogs and amphibians. A probit dose response model is being used to better estimate the potential for pesticides to affect nontarget organisms at the different concentrations expected in the field.
The Agency continues to evaluate the pesticide human health risk assessment process to identify still further improvements. Improvements in FY 2007 were the formation of the Toxicology Science Advisory Council (TOXSAC) and a new committee, Residues of Concern Knowledgebase Subcommittee (ROCKS). The TOXSAC began its meetings in October 2007. Its function is to provide guidance and advice to the human health risk assessment teams as they conduct their hazard assessment. The Council’s objective is to ensure that current standard practices and policies are consistently applied and that if deviations are warranted, the rationale for such departures is clearly and explicitly articulated in the hazard assessment portion of the risk assessment.
The ROCKS is a new committee composed of human health and environment risk assessors responsible for determining the residues of concern to be included in dietary risk assessments for food and water. ROCKS will evaluate toxicity, environmental fate, residue chemistry, and metabolism information in the risk assessment process. The ROCKS is anticipated to incorporate such tools as QSAR (quantitative structure activity relationship analysis) in the dietary risk assessment process and will foster increased collaboration across the pesticide regulatory program early in the risk assessment process.
The Dose Adequacy Review Team (DART) met to discuss dose selection for registrant conducted cancer studies for four pesticides during FY 2007. An agreement on doses before the studies are begun insures that the doses are adequate. In the past, detailed discussions about dose selection were necessary after the study had been conducted, and now, these discussions and the need to repeat studies have been eliminated.
Progress in Meeting Tolerance Reassessment and Reregistration Timelines
FY 2007 Accomplishments
During Fiscal Year 2007, the Agency completed a major milestone in the implementation of the Food Quality Protection Act by completing the tolerance reassessment program. EPA has made reassessment decisions for 100% of the 9,721 pesticide food tolerances that required reassessment under FQPA, and is well on it way to completing the remaining 27 reregistration eligibility decisions by October 3, 2008. In FY 2007 alone, the Agency completed 27 Reregistration Eligibility Decisions (REDs) and 84 tolerance reassessment decisions.
Status of Reregistration
To the end of FY 2007, the Agency has completed 357 REDs and must issue 27 more REDs to complete all reregistration eligibility decisions by October 3, 2008. EPA's goal is to complete the 27 remaining REDs during FY 2008, including the last 2 pesticides with associated food uses or tolerances. This will satisfy PRIA requirements and support the Agency's reregistration goals. EPA's schedule for completing these decisions can be found on the Agency's Website.
Status of Tolerance Reassessment
At the end of FY 2007, the Agency had completed a total of 9,721 tolerance reassessment decisions, addressing 100 percent of the 9,721 tolerances that required reassessment. EPA accomplished tolerance reassessment through both the registration and reregistration programs, by revoking tolerances for pesticides that have been canceled, by reevaluating pesticides with pre-FQPA REDs, and through other decisions not directly related to reregistration or registration.
Other Activities
FY07 - Use of Outside Reviewers
During FY 2007, the Agency continued its work sharing efforts with Canada’s Pest Management Regulatory Agency (PMRA), the Australian Pesticides and Veterinary Medicines Authority (APVMA), New Zealand, the European Union (EU) and the California Department of Pesticide Regulation (CDPR). The joint review/work sharing effort produced 5 new active ingredient registrations for conventional chemicals, one of which was the first trilateral joint review conducted by EPA, PMRA and APVMA. Four minor use actions were also completed in FY 2007 as part of the NAFTA joint review program. To date, 6 new active ingredients (conventional chemicals) are being jointly evaluated either by EPA and PMRA under NAFTA; or by NAFTA, the EU and New Zealand. Approximately 11 new active ingredient registration applications (6 conventional chemicals, 4 biopesticides and 1 antimicrobial) are expected to be submitted under the joint review program within the next 2 years (2008-2010). In addition, a total of 21 minor use submissions are expected to be evaluated under the NAFTA joint review program. In conducting joint reviews, EPA makes its own registration decision while sharing the study reviews and the risk assessment work and harmonizing its regulatory decisions with other national authorities.
EPA also continues to work with CDPR to expand its capacity to review residue chemistry studies and conduct dietary risk assessments in support of registration decisions. In FY 2007, CDPR reviewed the residue chemistry studies for six active ingredients and a total of 16 representative commodities or crops.
Contractors tasked with the review of hazard and exposure data continued to assist the Agency in the selection of endpoints and characterization of hazards for human health and ecological risk assessment. These contractor services enhanced the production of our risk assessments and contractor support was in general greater in FY 2007 than in the previous years of PRIA implementation. Contract support was obtained to reduce the time to complete data review particularly of acute toxicity studies submitted with antimicrobial applications.
As in FY 2006, during FY 2007, approximately 75% of the Pesticide Program’s active contracts or task orders/work assignments are performance based. Performance based contracts tend to be contracts with routine and predictable work assignments. Areas covered by these contracts include information management, records management, on-site computer leasing and support, outreach, and as appropriate, data review and risk assessment.Appendix A: Decision Review Times for Actions Completed During FY 2007
As required by FIFRA Section 33(k), the following table (an Excel file) provides the decision times for each decision (application) during FY 2007. Note that decision times indicated in red with an asterisk are decisions completed before the Agency received payment or a waiver was granted. Completion of a registration action before payment is received typically occurs in situations where a voluntary fee payment has been offered for an application that was pending with the Agency prior to March 23, 2004 (the PRIA effective date) or the Agency anticipates approval of a fee waiver based on past fee waiver approvals during the same maintenance fee cycle. Mandatory decision time frames changed for some PRIA action codes and fee categories between FY 2006 and FY 2007. A decision’s time frame is based on the fiscal year in which the application is received. Mandated time frames can be found in the fee schedule published in the Federal Register Notice on March 17, 2004 titled Pesticides; Fees and Decision Times for Registration Applications. The Agency’s target due date for completing a decision or action is based on 30 days in a month. The time frames specified in the Consolidated Appropriations Act of 2004 are in months. In the table, if the PRIA due date was met, while the Agency’s target date was not, a date was entered in the column labeled PRIA Due Date. As EPA improves its reporting capabilities, the Agency may update this table, as necessary.
Table of completed actions for FY 2007 (Excel, 276 KB) (Microsoft Excel Viewer![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20090510080842im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)