Introduction
Strategies for Outreach and Education
Ways to Educate Yourself
Ways to Educate Others, One-on-One
Ways to Conduct Community Outreach and Education
Ways to Work with the Media
Ways to Work with Hospitals, Medical Centers, and Universities
Appendix
Introduction
Given your interest in clinical trials, it is likely that you
already know quite a bit about how they work, the barriers associated
with them, and why they are important.
Whether you are a health care professional who wants to find ways
to reach your community, a member of a patient group who wants to
educate its members, or someone who wants to engage your group or
organization to initiate clinical trial outreach activities, this
guide can help you figure out avenues to support your activities. The
guide is not designed to be a comprehensive overview; rather, it
gives some examples of activities others have engaged in, and
provides tools and suggestions for implementation in your
community.
By informing your community about clinical trials, you can help people make
informed decisions about their cancer treatment or prevention
options, including the option of participation in a clinical trial.
The more people that participate in clinical trials, the faster we
can answer the critical research questions that will lead us to
better treatment and prevention options for all cancers.
This workbook is designed to complement the other materials in the
National Cancer Institute Clinical Trials Education Series. 1
Strategies for Outreach and Education
It is important to understand the reasons why so few adults with
cancer participate in clinical trials. A few are listed here.
Many people with cancer, or those at high risk for developing
cancer, are:
Unaware of the option of participating in clinical
trials. Research has consistently shown that most people are not
aware that clinical trials could be an option for cancer treatment
or prevention. Unwilling to go against physician's advice or direction.
Research has shown if a person's doctor does not recommend a trial
as an option for cancer treatment or prevention, he or she is very
unlikely to participate in one. Fearful, distrusting, or suspicious of research. For
many people, the idea of being "randomized" to one treatment makes
them feel they have less control over their care. Many are also
fearful of being "experimented upon" and not receiving treatment
for their cancer. Many people distrust those in the medical
community, based on past negative experiences or the historical
abuses of research participants. Unaware of clinical trials. The
reality or the perception that there are no trials in their local
community is a barrier for many people. Concerned about potential costs of trials. Some people who are insured
fear that their insurance company won't cover participation in a clinical trial.
Those who do not have insurance may worry about costs that are not covered by the
trial's sponsor. Facing personal or practical obstacles. There are many
costs, financial and otherwise, to participating in a clinical
trial. Time and travel that are required to seek care at a distant
trial site may be a concern for many people. The indirect costs of
being away from work and family may also be a concern. Finally,
some people may not wish to temporarily leave the care of their
physician to participate in a trial.
Many doctors are:
Unaware of clinical trials. Physicians are not
always aware of available clinical trials. Some may not be aware of
the local resources or may assume that none would be appropriate
for the people they treat. Unwilling to "lose control" of patient's care. Most
doctors feel that relationships with the people they care for are
very important. They want what is best for each person. Some
doctors fear that if a person must be referred elsewhere to
participate in a trial, they may lose control of the person's
care. They may not understand that every effort is made to
maintain the physician relationship, even when a person is on a
trial. In addition, many doctors may fear the loss of income if a
person is referred elsewhere for his or her cancer care. Under the impression that standard therapy is best. Many
physicians may not adequately understand how clinical trials are
conducted or the importance of clinical research. Some physicians
believe that the treatment in clinical trials is not as good as
the standard treatment they might provide to people. They also may
be uncomfortable admitting that there is uncertainty about which
treatment is best in a phase 3 clinical trial.
Additional Clinical Trial Barriers for Ethnically Diverse Populations
There is long-standing fear, apprehension, and skepticism in minority populations about medical research due to real abuses that have happened in the past (e.g., the legacy of the Tuskegee syphilis study). Among these populations, there is often widespread fear and distrust of the medical care system as a result of discrimination, indifference, and disrespect. Some may feel that they do not want to give up their rights by participating in a trial, or lose their power by being "experimented upon." Others may be skeptical about the quality of care that would be provided in a clinical trial. Some may find that trial recruitment strategies are not sensitive to their needs.
Doctors may not mention clinical trials as an option for cancer care.
As noted above, many physicians do not refer their patients to clinical trials. However, some physicians may avoid suggesting a clinical trial to their minority patients, out of concern that patients would see him/her as insensitive. Moreover, some physicians may unwittingly discriminate against older patients, or those who are from certain ethnic or cultural backgrounds.
Many people may face additional problems accessing clinical trials. Depending on where they live or their access to transportation, people may have difficulty getting back and forth from a clinical trial site. Those with low income may find it difficult to take time off work or find appropriate childcare. Other barriers, such as a lack of health insurance or lack of general health care, clearly present difficulties in accessing trials.
Cultural or ethnic backgrounds may include values and beliefs that are very different from Western Medicine. Many people have cultural beliefs that Western medicine cannot address their health concerns. Different ethnic and cultural views of health and disease (e.g., fatalism, family decisions about treatment, use of traditional healers, prayer, herbal medicines, or use of complementary/alternative health practices) may make clinical trials a less attractive treatment option. For prevention trials, many may feel that the risk of a potential disease and its consequences may be less important than meeting daily needs.
Language and/or literacy barriers may make it difficult for some people to understand and consider participating in clinical trials. The complexity of forms, including informed consent documents, may also be a barrier to those considering participation in a clinical trial. Translation can also be difficult if the person translating information has not had specialized training.
One of the biggest hurdles for clinical trial education is
overcoming suspicion of medical research.
It is important to note that strategies for clinical trial
outreach and education will vary, based on the type of trial and its
requirements for participation. Although some of the following
strategies were designed for cancer prevention trials, many may also
be used for other types of clinical trials.
Strategy 1
Educators should be familiar (and preferably a part of) the
communities they are trying to reach. People who are known, trusted,
and accountable will be more effective and more believable when
discussing clinical trials with community members.
Suggested Steps
Use easy to understand language. In some cases
this may mean using a community's first language. Involve people from the community, especially community
leaders. Find ways to develop collaboration and encourage
ownership in the outreach program.
|
Tip
If you are not from the population(s) you seek to work
with, it is critical that you or your organization develop
meaningful collaborative partnerships with organizations
within those communities.
|
Strategy 2
Address important concerns and perceptions, benefits, and risks
about clinical trials through one-on-one contact. One-on-one contact
is one of the best ways to educate others.
Suggested Steps
Make sure you do not judge someone's values if
they are different from your own. Find ways to present information
that complement the values someone holds. Address risks and costs in a frank, open, and honest
way. Stress the importance of enrollment in trials to the
family and to future generations. Stress the importance of equal access to the highest
quality care, including clinical trials. Each person has the right
to know and understand every option available with regard to his
or her health care. Promote the balance of spirituality, faith, medicine,
and science. See the next section for suggested messages for specific
ethnic/ racial groups.
Strategy 3
Discuss potential benefits of participating in a clinical trial;
but do not overlook the risks.
Suggested Step
Discuss the fact that because people are monitored closely under
clinical trial protocols, they often receive a higher quality of
medical care and follow up than do those who are not enrolled in
clinical trials.
Strategy 4
Avoid disrupting home and work schedules when conducting education
or outreach activities.
Suggested Steps
|
Key Points for Outreach and Education
Clinical trial outreach strategies must
incorporate an understanding of a potential participant's
decision-making process, his or her culture, family and work
life, and economic concerns. Those conducting education and
outreach must find ways to present clinical trial
information that complement the values people in the
community hold. Education and outreach strategies should stress
the importance of equal access to the highest quality care,
including clinical trials. Each person has the right to know
and understand every health care option available. Clinical trials must be explained in a way that is
respectful and easy to understand, addresses someone's fears
and concerns, and addresses risks and benefits. The research team must ensure that the informed
consent process truly reflects a participant's understanding
of the risks and benefits of the clinical trial. Involving
family, members from the participant's community, and
culturally competent staff are some ways to help verify that
the participant has received the information in a way that
he or she can understand and made the decision to
participate voluntarily.
|
Research has shown that there are many differences in who gets
cancer among people of different races, ethnicities, and
socioeconomic backgrounds. Certain racial and ethnic groups, as noted
on the pages that follow, are also more likely to die of cancer than
other groups. These differences may be due to a variety of reasons,
such as late stage of disease at diagnosis, barriers to health care
access, history of other diseases, biologic and genetic differences
in tumors, health behaviors, and the presence of other risk factors
for cancer. In addition, some cancers that have a high rate of
developing in one ethnic group are rare and may not be listed among
the top 10 cancers in the U.S. population as a whole.* Because rare
cancers may not receive as much
attention as those in the "top 10," it is even more important for
people of particular ethnic and cultural groups to be aware of
research so that they can work to find
ways to decrease the burden of these unusual cancers in their
populations.
Differences in cancer screening and treatment have also been
documented for people of different ages, as well as those from
different socioeconomic, educational, and racial/ethnic
backgrounds.
*Based on the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program. SEER is
the most authoritative source of information on cancer incidence and
survival in the United States.
Information on data sources used in this section:
Cancer incidence data come from the NCI SEER program, covering 14 percent of the U.S. population.
Cancer mortality data come from the National Center for Health Statistics (NCHS) and
covers the entire U.S. population. Cancer incidence and death rates for some racial and ethnic
populations may be limited by problems in ascertaining race and by the misreporting of race and ethnicity on forms used to collect information on cancer incidence, deaths, and the populations at risk. For instance, while reporting race for African-American and White populations is generally considered reliable, biases are more serious for smaller populations, particularly American Indian/Alaskan Natives, as well as for groups living in smaller geographical areas. Additionally, it is important to note that Hispanics can be of any race and are not mutually exclusive from White, African Americans, Asian/Pacific Islanders, and American Indian/Alaskan Natives. These biases can affect trends and comparisons among groups.
Ethnically Diverse Populations-Some Definitions
Diverse populations include minority, ethnic, and racial groups
designated by the U.S. Government, including:
American Indian or Alaska Native Asian American Black or African American Hispanic or Latin American Native Hawaiian or other Pacific Islander
Ethnically diverse populations are growing rapidly, and according
to the 2000 Census, about 25 percent of the U.S. population reported
their race as something other than White.
About 17 percent of the U.S. population over age 5 (more than 44 million people)
do not speak English at home. Of these,
NCI's working definition of diverse populations also includes
medically-underserved populations, such as rural, low-income, and
low-literacy level individuals of any racial or ethnic group.
Medically underserved populations are those that lack easy access to,
or do not make use of, high-quality cancer prevention, screening and
early detection, treatment, or rehabilitation services. In general,
these groups experience higher cancer death rates than the U.S.
population as a whole.
Outreach Strategies
The strategies listed below are not meant to be a complete
overview of barriers and strategies; nor should the information be
generalized to all people in these groups. Attitudes within various
populations vary greatly, depending on a person's age, socioeconomic
status, community, and other factors. The broad outline here provides
some background, context, and potential strategies for potential
education and advocacy efforts.
|
Participation of Different Groups in
NCI Treatment Clinical Trials2
The percentage of White patients enrolled in NCI clinical trials parallels that of the overall U.S. population. However, different patterns are seen for Black, Asian American, and Hispanic cancer patients.
Black children and young adults have accrual to clinical trials comparable to their White peers, as do Black women.
Asian American and Hispanic children and young adults have accrual to clinical trials comparable to their White peers.
The percentage of Black men who have cancer and are 30 to 59 years old who participate in clinical trials is markedly lower than the percentage of White men with cancer in this age group.
The percentage of Asian Americans and Hispanics aged 30 to 80 that accrue to clinical trials is less than that of Whites.
In terms of age, those 80 years of age or older are least likely to be enrolled, followed by those aged 30 to 39 years.
|
Patient accrual for NCI-sponsored cancer treatment trials by sex and race/ethnicity
2
| Race/Ethnic Group |
Males (%) |
Females (%) |
Percentage of U.S. Population |
| White |
36 |
46 |
72 |
| Black |
4 |
5 |
12 |
| Hispanic |
4 |
3 |
12 |
| Asian |
1 |
1 |
4 |
| American Indian |
0.5 |
0.5 |
1 |
| Hawaiian/Pacific Islander |
0.5 |
0.5 |
0.5 |
| Total |
44 |
56 |
100 |
Percentage of estimated United States female population represented in NCI-sponsored
cancer treatment clinical trials: log scale (by age and race/ethnic group)2
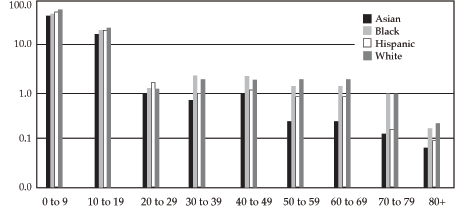
Percentage of estimated United States male population represented in NCI-sponsored
cancer treatment clinical trials: log scale (by age and race/ethnic group)2

African Americans and Clinical Trials
Background
A person who is Black or African American has origins in any of
the Black racial groups of Africa. This definition includes:
Cancer in African Americans
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999.)3
Overall, African Americans had the highest risk of any U.S.
racial/ethnic group of not only getting cancer, but also for dying from cancer.
African American Men:
Of all ethnic and gender groups, African American men have the highest overall rate of having cancer.
Of all ethnic and gender groups, African American men have the highest overall rate of dying from cancer.
Of all men, African Americans have the highest incidence and death rates from many cancers, including lung and bronchus, prostate, and colon and rectum cancer. In addition, in 2001, African American men were at least 50 percent more likely to get prostate cancer than were men of any other ethnic group.4
Top five cancers: Prostate, lung and bronchus, colon and rectum, oral cavity and pharynx, and stomach
African American Women:
Of all women, African Americans have the highest incidence rates for colon and rectal cancer, lung and bronchial cancer, and pancreatic cancer.
Of all women, African Americans have the highest death rates from many cancer, including breast, colon and rectal, pancreatic, uterine, and cervical cancers.
While they have the second highest rates of all women of getting breast cancer, they have the highest rate of dying from the disease.
Top five cancers: Breast, colon and rectal, lung and bronchial, uterine, and pancreatic
Challenges
The legacy of the Tuskegee Syphilis Study (in which researchers
studied but did not treat African American men with syphilis) has
contributed to long-standing mistrust in African American communities
concerning clinical research. Widespread skepticism about the medical
care system exists as a result of a long history of discrimination,
indifference, and disrespect. The oral history contributing to this
mistrust is particularly important to recognize.
Some African Americans may believe that if they agree to
participate in a trial, they will not be appropriately cared for, nor
honestly informed of the risks or the benefits. They may fear
that:5
Placebos would be substituted for lifesaving
interventions Treatments that work would be deliberately withheld They would not receive a full course of treatment,
especially if funding sources for the clinical trial were no
longer available
Other cultural beliefs and attitudes that affect research
participation include hopelessness, fatalism, and doubt about the
usefulness of cancer prevention and control. Faith, folk
remedies, and the role of the family are other important influences
for African Americans.6
Clinical trials may be a lower priority to African Americans and
others who have a low income, less access to transportation and
health care, less information about clinical trials, and low levels
of literacy. Concerns about family and work responsibilities may also
be a significant barrier.7 African American men have
noted concerns about researchers not giving back to the community,
being uncomfortable talking about prostate cancer, and past negative
experiences with the medical care system.8
Potential Solutions
Cultural strategies:
Find people who are already active in
organizations to help spread the word about clinical trials.
People who are known, trusted, and accountable in the community will be better
messengers than will outsiders. Explore partnerships with African American churches,
particularly for health issues central to the mission of the
church. Faith is a very important part of many African American
cultures and the most successful outreach efforts usually involve
churches that have two or more paid clergy, and medium or large
memberships. Ensure that the informed consent process truly reflects
the participant's understanding of the risks and benefits of the
clinical trial. Involving family, members from the participant's
community, and culturally competent staff are some ways to help
verify that the participant has received the information in a way
that he or she can understand and made the decision to participate
voluntarily.
Program strategies:
Talk about trials using one-on-one
contact-preferably with another African American person-through
churches, schools, civic organizations, and African American
sororities and fraternities. Word of mouth can be an effective
way to reach others. Conduct in person outreach to complement other education
efforts using videos, brochures, or advertisements. Present real-life situations that exemplify statistics
or written messages. Effective dialogue can take place through a
church-sponsored forum or an educational session that allows for
open discussion and questioning. Involve local celebrities, including DJs at African
American radio stations, by asking them to share messages about clinical
trials. Ask newspapers and local media to join in education
efforts. Provide personal, "real world" discussion of clinical trials and
follow up in any education program.
Key Messages
Distrust of medical research is a critical issue, but it is
important that African Americans be presented with an opportunity to
find answers to research questions. The prospect of learning
information that will help all people access treatment and better
manage disease can be a powerful motivator.
Some African Americans may be more interested in clinical trials
if they understand that participation means they:
Contribute to their community and their families Join a group of people like themselves nationally, and
in their local community
Clinical trial educational messages need to include information on
the following topics:
Severity of the cancer problem nationwide Underlying myth that African Americans don't need to be
concerned about cancer Toll that cancer (especially breast and prostate) is
taking on African American women and men-and that we don't know
why Prevention and treatment options for high-risk African
Americans-and the need for more research Importance of clinical trials-and what it means for all
people if all groups are not represented in a trial Laws on participant protection and rights What risk means for the individual and others in his or
her community
Asian Americans and Clinical Trials
Background
A person who is Asian has origins in any of the original peoples
of the Far East, Southeast Asia, and Indian subcontinent. The term
"Asian" refers to persons from the following and other Asian,
Southeast Asian, and South Asian backgrounds:
- Chinese
- Vietnamese
- Pakistani
- Filipino
- Cambodian
- Thailandian
- Japanese
- Hmong
- Indian
|
- Korean
- Laotian
- Bangladeshi
- Indonesian
- Sri Lankan
- Nepalese
- Bhutanese
- Sikh
- Burmese
|
|
"Asian Americans" and "Pacific Islanders" are two discernibly distinctive groups, comprised of numerous heterogeneous ethnic subpopulations. These broad categories fail to show mortality rates that, in some instances, strongly differ among ethnic groups. Whenever possible, this publication separates out these groups.
|
U.S. residents who reported they were Asian* make up 4.2 percent of the total population. Chinese is the leading Asian group (2.7 million) followed by Filipino (2.4 million) and East Indian (1.9 million).1
*Self report, alone or in combination with one or more other races
The Asian population includes many groups who differ in language, culture, and length of residence in the United States.
Some of the Asian groups, such as the Chinese and Japanese, have been in the United States for several generations and often have
literacy, education, and socioeconomic characteristics that are above the national average. On the other hand, groups such as the Hmong, Vietnamese, Laotians, and Cambodians are comparatively recent immigrants, and tend to have limited acculturation and poverty rates below the national average. It is important to note that 88 percent of Asian Americans and Pacific Islanders (AAPI) are either foreign-born themselves or have at least one foreign-born parent. Of those who speak Asian and Pacific Island languages at home, more than 22 percent say they speak English "not well" or "not at all."1
Cancer in Asians
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999).)3
Some Asians are much more devoted to traditional medical practices
than to Western medicine. For example, a study of breast and cervical
cancer screening in Chinese women found that more than 2/3 had gone
to traditional providers for preventive health care, went to temples
to pray for their health, and looked to fortune-tellers for
guidance.9 Another study of cervical cancer screening in
Cambodian women indicated beliefs that fate cannot be changed by
detection, cancer is incurable, and cancer will not develop if
traditional practices are used.10
Like the Hispanic/Latino populations, Asian/Pacific Islanders experience lower incidence and mortality rates overall compared with other minority groups. However, they do experience higher incidence and mortality rates for certain cancers. Based on three-year averages, more than 18 percent of Asian and Pacific Islanders lack health insurance.
Asian/Asian Pacific Islander men:
Of all men, AAPIs have the highest incidence rates of liver and stomach cancer.
Of all men, AAPIs have the highest death rates from liver cancer.
Asian/Asian Pacific Islander women:
Seventy percent of Asian Americans come from countries with the world's lowest overall rates of breast cancer, yet after living in the U.S. for as little as 10 years, Asian women have an 80 percent higher risk of getting the disease than recent immigrants.
In addition, third and fourth generation Asian American women have rates of developing breast cancer that are similar to their neighboring Caucasian women.11
Asian American women in general have the lowest rates of Pap test, mammogram, and breast exam screening of any ethnic group.6,11
Of all women, Asians have the highest rates of liver and stomach cancer.
Of all women, Asians have the highest death rates for liver and stomach cancer.
|
Cancer in Specific Asian Groups
The NCI SEER cancer incidence rates noted in this publication are available for different levels of racial/ethnic detail in each of the time periods (1988-1992 and 1992-1999).
Although the SEER program routinely collects detailed racial/ethnic information on the cancer patients in its coverage areas, the lack of comparable detail in the racial/ethnic county-level population estimates from the U.S. Census Bureau means that incidence rates for certain racial/ethnic groups can be calculated only for time periods centered on the decennial census. For example, county-level census information for Asian and Pacific Islanders by subgroups (Chinese, Filipinos, Hawaiians, etc.) is still only available from the 1990 Census.
|
From 1988 to 1992, Chinese, Japanese, Korean, and Vietnamese* groups all had higher rates of getting liver and intrahepatic bile duct (IHB) and stomach cancers than Whites, and Chinese and Japanese had higher mortality than Whites from these two cancers, as well.12 In this same time period, Korean and Vietnamese women had higher incidence rates of cervical cancer than White women.12
*Liver and IHB not calculated for Vietnamese women
From 1988 through 1992, the top five cancers for many Asian groups
are as follows:12
Women:

Men:

Challenges
Values in many Asian cultures may be different than the
Euro-American system, with decisions reached by consensus, group
welfare being of primary value, and individual life not as sacred.6
In some Asian cultures, the family is responsible for treatment
decisions and the patient is not told of his or her diagnosis.
Many in the Asian immigrant communities need a lot of support as
they learn about clinical trials, and they need to feel safe asking
questions. "Saving face" in public is important. In many cases, Asian immigrants
may feel it is disrespectful to ask questions of doctors or health
professionals.
Recent immigrants also may be dealing with a combination of
educational, social, and health problems, along with emotional
difficulties related to separation and isolation. Many are in
low-wage jobs and need to get permission to take time off work to
take care of health care needs.
The language barrier also is difficult to overcome. Many Asians do
not speak English, and this may not be readily apparent. There are so
many Asian languages that deciding on the language(s) in which
information should be printed is difficult. This barrier is
particularly important in issues surrounding informed consent. The
informed consent process is intimidating for all people and is
especially so for those with limited English skills.
Potential Solutions
Cultural strategies:
Assess how long the group you are trying to reach
has been in this country as well as the countries of origin represented. It is
important to fit the educational outreach to the culture and to
use people from the communities to reach community members. Involve family members in learning about the risks and
benefits of clinical trials, but resist using them as translators of medical information. In particular, avoid having children-who may be more proficient in English than their parents-serve as translators. It is preferable to enlist someone who is trained for this work. Ensure that the informed consent process truly reflects
the participant's understanding of the risks and benefits of the
clinical trial. Involving family, members from the participant's
community, and culturally competent staff are some ways to help
verify that the participant has received the information in a way
that he or she can understand and made the decision to participate
voluntarily.
Language strategies:
Find respectful ways to make sure that information
is being understood. Someone who does not understand English may
say "yes" or nod, even if they do not understand what is being
said. To ensure that the person you are talking with understands what you have said, staff may ask, "Many people have a hard time understanding information like this. I want to make sure that I explain it clearly. Could you please tell me, in your words, what I have just said." When translating materials, at least two bilingual, bicultural translators should be used. In addition, materials should be pilot-tested with the target audience.
Program strategies:
Use health fairs, which have been successful when
organized by community members, and involve the leadership of
existing Asian groups in clinical trial outreach. If possible, translate clinical trial information to be
used in ethnic newspapers. Many immigrant groups get their health
information from press that is printed in their own languages. Invite a professional to deliver information about
clinical trials. Preferably, a doctor or nurse from that community
should be invited, who can encourage others to ask questions.
Often, laypeople do not have much credibility as an information
source. Keep workshops or programs short (30 to 40 minutes) because many new immigrants
have more than one job, in addition to other responsibilities. While recent immigrants may not have been concerned with breast cancer in their countries of origin, it is important to educate these women about their increased risk for this disease.
Key Messages
A family's receptivity to
cancer treatments and trials will depend on the experience that
relatives have had with the medical system. For example, if a relative did not
survive cancer, it may be taboo to talk about him or her. Remember
to consider feminine modesty and traditional gender role values.
Native Hawaiians and Other Pacific Islanders and Clinical
Trials13
|
Native Hawaiians and Pacific Islanders are often
aggregated nationally into the "Other" category or currently
the "Asian American/Pacific Islander or AAPI" category.
It is important to note that these are two discernibly distinctive groups, comprised of numerous
heterogeneous ethnic subpopulations.
These groupings have obscured disparate mortality rates
that are prevalent in one group and not the other. Whenever possible, this publication separates out these groups.
|
Background
Native Hawaiians and other Pacific Islanders of Polynesian,
Micronesian, and Melanesian ancestry* made up 0.3
percent of the total U.S. population.1
This group comprises
more than 25 diverse groups with variations in historical
backgrounds, languages, and cultural traditions.
*Alone or in combination with one or more races.
Among Pacific Islanders in the United States, Native Hawaiians are
the largest group, 58 percent (211,014). Three-fourths of Pacific
Islanders live in the states of California and Hawaii and they are a
relatively young population, with a median age of 25 years and an
average family size of 4.1.
The term "other Pacific Islanders," refers to the peoples of
Polynesia, Micronesia, and Melanesia, and includes:
- Chamorros
- Samoans
- Fijians
- Tongans
- Tahitians
- Marshallese
|
- Chuukese
- Kosraen
- Yapese
- Pohnpeian
- Palauan
- Other Pacific Islanders
|
Within this group are six U.S.-associated Pacific Island
jurisdictions--the Federated States of Micronesia, the Republic of
Palau, the Republic of the Marshall Islands, Guam, American Samoa,
and the Commonwealth of the Northern Marianas-that have various
political relationships with the U.S.
The population of the U.S.-associated Pacific Island jurisdictions
is approximately 427,000. The health status varies within and among
the jurisdictions, but is generally worse than for Americans. The
jurisdictions must contend with health conditions found in both
developing countries (e.g., malnutrition, dengue fever, cholera, and
tuberculosis) and developed countries (e.g., diabetes, heart disease,
and cancer).
Cancer in Native Hawaiians
Hawaiian Men:
(For the years 1988-1992)12
Have the second highest cancer mortality,
behind only African Americans
Of all men and of all ethnic groups,
Top five cancers: Lung and bronchus, prostate, colon and rectum, stomach, and non-Hodgkin's lymphoma
Hawaiian Women:
(For the years 1988-1992)12
Top five cancers: Breast, lung and bronchus, colon and rectum, uterine, and stomach
Cancer in Pacific Islanders
Cancer surveillance and databases are rudimentary or non-existent
in most of these jurisdictions, rendering cancer rates unknown. What
is known is that cancer is among the top three causes of death. The
most commonly reported cancers for males were cancers of the lung and
prostate, and for females, cancers of the breast, cervix, and
lung.
Challenges
A history of oppression, higher prevalence of
behavioral risk factors, ineffective cancer prevention and control
efforts, and poor access to state-of-the-art services for cancer
prevention, early detection, and treatment (including low
representation in clinical trials) contribute to increased cancer
risk and mortality among Native Hawaiians and other Pacific
Islanders. Many Native Hawaiians and Pacific Islanders are
socio-economically disadvantaged and underserved in terms of
access to health and social services. In the U.S., many Pacific Islanders do not speak English
at home. There is a general distrust of research among island
communities. This distrust can also have negative consequences for
those participating in a trial, such as poor compliance or
avoidance. Geographic barriers are a problem for many Pacific
Islanders; clinical trials are unavailable for most Western
Pacific and Samoan communities and rural Hawaiian communities. The conduct of many clinical trials lacks cultural sensitivity
and does not address language needs; it also does not interpret cultural
behaviors and preferences.
Potential Solutions
Cultural strategies:
Tailor the educational outreach to the culture and
use "cultural brokers" (members of the community) to reach other community
members. Include the family unit, which for many Native Hawaiians
and Pacific Islanders includes extended family members and
friends, when educating about the risks and benefits of clinical
trials. The role of the woman is central to the family in many
Pacific Island cultures. Ensure that the informed consent process truly
represents the participant's understanding of the risks and
benefits of the clinical trial. Involving family, members from the
participant's community, and culturally competent staff are some
ways to help verify that the participant has received the
information in a way that he or she can understand and made the
decision to participate voluntarily.
Program strategies:
Use personal contacts through a family member or a
friend to do education and outreach. Explain the benefits of research to the community at
large. Address issues of medical care that are not covered by
clinical trials, as these are an important concern for
participants who may not have insurance or are underinsured.
Native Americans and Clinical Trials14,15
Background
A person who is considered Native American has origins in any of
the original peoples of North or South America (including Central
America) and maintains tribal affiliation or community
attachment.
The term "Native American" refers to:
American Indians Alaska Natives:
Native Americans are made up of culturally distinct and diverse
communities. The U.S. contains 511 federally recognized tribes, with
Native American people living in every State. The largest tribes are
Cherokee and Navajo. More than 9 percent of the U.S. population
reported American Indian or Alaska Native status in the 2000
Census.
Indian Health Service
There are more than 300 hospitals and health clinics, located on
or near Indian reservations, run by the Indian Health Service (IHS).
In recent years, many tribes have assumed management of some of these
health care facilities.
The Indian clinics and hospitals are unable to provide the
high-tech medical care needed to diagnose and treat cancer. For this
reason, the IHS Contract Health Services program pays for Indian health
care provided by non-IHS providers. However, this program is
chronically short of funds. Depending on the region/tribe, its local
priorities, and funding remaining in the service contract, certain
treatments may not be available. At present the Contract Health
Services does not reimburse for many treatments that are deemed
"experimental," which keeps many Indian people out of clinical
trials.
Although 54 percent of Native Americans live in urban areas, less
than 2 percent of the IHS budget is spent in urban
clinics.6 These clinics are severely underfunded and must
rely on other sources of support, including Medicaid revenue. Many
urban Indians choose to return to their home reservations for
care.
Based on 3-year averages, American Indians and Alaska Natives were the least likely of the major racial groups to have health insurance.1A
Cancer in Native Americans
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999.)3
While American Indian/Alaska Natives experience some of the lowest cancer rates among all ethnic groups, they do experience higher incidence and mortality for certain cancers. Cancer is the second leading cause of death among Native Americans
and is the leading cause of death among Alaska Native
women.16,17
American Indian/Alaska Native Men:
Top five cancers: Prostate, lung and bronchial, colon and rectal, kidney and renal pelvis, and stomach
American Indian/Alaska Native Women:
Top five cancers: Breast, colon and rectum, lung and
bronchial, uterine, and ovarian
|
Alaska Natives
(For the years 1988-1992)12
Alaska Native men had the highest rates of getting colon and rectal cancer among all ethnic groups.
In this same time period, Alaska Native women had the highest rates for getting and dying from colon, rectal, and lung cancer among ethnic groups, and their rates of getting cervical cancer were twice as high as those for White women.
The top cancers:
Men: Lung and bronchus, colon and rectum, prostate, and stomach
Women: Breast, colon and rectum, lung and bronchus, and cervical
American Indians (Living in New Mexico)
American Indian men had the highest rates of all ethnic groups of getting kidney cancer. In this same period, American Indian women had higher rates of cervical and ovarian cancers than the U.S. White female population.
Top cancers in American Indians:
Men: Prostate, colon and rectal, kidney and renal pelvis, and lung and bronchus
Women: Breast, ovarian, colon and rectal, gallbladder, and uterine
|
Challenges
A history of disrespect, racism, and poverty has
contributed to a distrust of science and research by Native
American populations. To protect the interests of Native American people, many
tribes have their own Institutional Review Board (IRB), in
addition to those required by Indian Health Service (IHS) or
tribal facility. Successful clinical trial outreach requires that
the investigator work closely with the tribal IRB in addition to
those of the IHS. Native Americans are a culturally distinct and diverse
community and their beliefs about cancer, and experiences with
diabetes, alcoholism, poverty, and traditional roles can
significantly affect the success of clinical trial educational
programs. On average, Native Americans are younger, have a lower
rate of high school completion, and have higher rates of poverty
and unemployment than Whites.6 Many Native people do not have access to quality health
care. Many tribes do not have a word for cancer in their
languages and historically, the disease was thought of as
something that affected only the "White man." Some Native
Americans may hold a fatalistic attitude toward cancer, and fear
that if they talk about cancer or even think about it, they might
catch it. It is unlikely that Native Americans would participate
in a cancer trial without hearing messages from other Native
Americans, yet there are few cancer survivors to serve as role
models. Informed consent forms and procedures may serve as a
barrier to recruitment because the language used in such forms may
not be well understood. Because of other pressing health issues, such as
diabetes, obesity, and substance abuse, in addition to extreme
poverty, cancer screening and treatment may not be as important to
some Native people. There may be a tribal taboo on the loss of body parts
that needs to be discussed in relation to clinical trials. Transportation is an important barrier for Native
Americans who live in rural areas. Differences in communication styles are important to consider.
Many Native Americans are reserved, reluctant to ask questions, or
don't discuss their health problems. Body language also is
important, with respect for personal space and friendly gestures
such as smiling and eye contact being key. Traditional roles are such
that women are usually caretakers and often place their needs
last. In addition, Native American women value modesty and
privacy, and many traditional Native American couples find a male
health care provider for the woman unacceptable.
Potential Solutions
Cultural strategies:
Use group activities such as sharing and caring
for others because they are universal concepts among Native
people, and should be a part of any clinical trial education
program. Incorporate the use of traditional healing ceremonies as
well as spiritual connections, which can be very important for
people in these communities. Family plays a central role in American Indian life. The
needs of the family may take precedence over the needs of the
individual. When appropriate, the patient's family should be
involved in the decision-making process. Ensure that the informed consent process truly
represents the participant's understanding of the risks and
benefits of the clinical trial. Involving family, members from the
participant's community, and culturally competent staff are some
ways to help verify that the participant has received the
information in a way that he or she can understand and made the
decision to participate voluntarily.
Language strategies:
Use easy-to-understand language and a gentle
approach to education and outreach. Try to include materials that
portray Native Americans. Make sure that patient consent forms are understood.
Reading out loud or encouraging consultation with others may be
important.
Program strategies:
Emphasize that participation in a trial can help
improve cancer care for the next generation. Use stories and visual tools that focus on the
family. Use one-on-one or small group education and outreach
techniques to respect privacy. Work with community or tribal elders such as community
health representatives and public health nurses, to find out the
best ways to conduct outreach and education efforts. Find out if transportation is needed to get to the site. Use public service announcements on Native American
radio.
Key Messages
Messages should be culturally relevant and discuss
issues related to family and community. Tribal beliefs are very diverse and programs should be
designed on a site-specific basis with the help of tribal
advisors.
Hispanics and Clinical Trials
Background
The terms "Hispanic" and "Latino" refer to people born in North,
Central, and South America, and in the Caribbean whose language is
Spanish. Someone who is Hispanic or Latino is a person of Mexican,
Puerto Rican, Cuban, Central or South American, or other Spanish
culture or origin, regardless of race.
In the mainland United States today
the largest groups within the Hispanic community are:
Mexican (58.5%) Puerto Rican (9.6%) Central American (4.8%) (Salvadorian, Guatemalan,
Honduran, Nicaraguan, Panamanian, and other people from countries
in Central America) South American (3.8%) (Colombian, Ecuadorian, Peruvian,
Argentinean, Venezuelan, Chilean, and other people from countries
in South America) Cuban (3.5%)
The Hispanic population is the fastest growing ethnic group in the
U.S. In the 2000 Census, Hispanics eclipsed African Americans to
become the second largest ethnic group, with 12.5 percent of the
population reporting Hispanic or Latino status. Hispanics as a group
comprise many different races and ethnicities. Within these
subpopulations, other differences exist according to culture,
beliefs, lifestyles, and experiences, but Hispanics agree that
certain commonalities go beyond specific nationalities. In general,
the U.S. Hispanic population is younger, with more people per
household, and has lower rates of employment, less education, and
lower economic status than do Whites.6
There are 28 million U.S. residents aged 5 and older who speak Spanish at home-about 10 percent of the U.S. population. These percentages vary greatly throughout the United States; for example, states like California, Texas, and New Mexico, have approximately 30 percent of residents who speak languages other than English.1 Among all those who speak Spanish at home, almost half speak English "less than very well." It is important to note that in 2000, 68 percent of the U.S. population is foreign-born or had at least one parent who was foreign-born.1
Cancer in Hispanics*
(Unless otherwise noted, all data is age-adjusted incidence or mortality rates between 1992 and 1999.)3
*"Hispanic" is not mutually exclusive from Whites, African American, Asian/Pacific Islanders, and Native Americans.
Although Hispanics had the largest reduction in cancer mortality
rates of any U.S. ethnic group (-1.6 percent) they also had the greatest number of uninsured
people. Based on three-year averages, more than 33 percent of all Hispanics lack health insurance coverage.1a A recent study showed that uninsured
Hispanic women are more than two times more likely to be diagnosed
with breast cancer at a later stage than other women, and uninsured
Hispanic men are almost four times more likely to be diagnosed with a
later stage of prostate cancer than non-Hispanics.19
While Hispanics/Latinos have lower incidence and death rates overall compared with those of African Americans and Whites, they do experience higher rates for certain cancers.
Latino/Hispanic men:
Top five cancers: Prostate, lung and bronchus, colon and rectal, non-Hodgkin's lymphoma, and stomach
Latina/Hispanic women:
Top five cancers: Breast, colon and rectal, lung and bronchus, cervical, and uterine
Challenges20
Many Hispanics have strong religious and cultural
beliefs. Some may believe strongly in "fatalismo" (fatalism) and
"resignación" (resignation)-that diseases or illness cannot
be controlled because they are inherited. Many may use folk remedies [such as "uña de
gato"(cat's nail)] to treat cancer or wait until they are in
serious pain to see a doctor. In one study, barriers that were identified by Latinas
considering a cervical cancer trial included transportation, fear
of getting a placebo, care of children and family, and care from a
male provider.7 Although Spanish is one language, regional dialects need
to be considered when translating materials. The language barrier is particularly important in issues
surrounding informed consent. The informed consent process is
intimidating for all people and is especially so for those with
limited English skills. Some Hispanics think that cancer treatment will only
prolong life but that no effective cure exists for the disease.
Even if people are treated, it is felt that the type of treatment
depends on the person's ability to pay. Hispanics may not obtain health care until they are very
sick and cannot perform normal functions. This adds to the number
and severity of health problems that need to be evaluated.
However, women are more likely than men to seek medical care. Many in the Hispanic community feel that doctors do not
communicate well with them and they do not feel well informed
about trials. Many hold the perception that doctors may have
financial interests in a trial, and there is a lack of trust
around participating in scientific research. A sense of fatalism and resignation is usually strongest
in women and older men. This may make people feel that treatment
is useless. Machismo is a barrier because men feel they are the
family protectors and should not show weakness. Hispanics' biggest barriers to accessing health care are
money, time, and language. Other common problems include lack of
insurance, problems with transportation or childcare, and getting
off work to see a doctor. Although face-to-face interaction is important,
Hispanics would not welcome unknown health educators or volunteers
into their homes. Men lack trust in a Federal Government source,
while most women tend to trust it.
Potential Solutions
Cultural strategies:
Collaborate with people who are from the
communities and speak Spanish. Community members can identify with
people who have a direct tie to their situation. Personal
interaction is very important. Testimonials from local pastors,
Hispanic celebrities, or doctors who have experienced cancer
themselves are beneficial. Involve family members in learning about the risks and
benefits of clinical trials. Sometimes children have learned to
speak English more quickly than their parents, so they can be
helpful in translating forms and brochures. It is important to
note, however, that using children as translators has both pros
and cons. It is preferable to use someone who is trained for this
work. Ensure that the informed consent process truly reflects
the participant's understanding of the risks and benefits of the
clinical trial. Involving family, members from the participant's
community and culturally competent staff are some ways to help
verify that the participant has received the information in a way
that he or she can understand and made the decision to participate
voluntarily.
Language strategies:
Find respectful ways to make sure that information
is understood. When people do not understand English, they may say
"yes" or nod, even if they do not understand what is being said.
To ensure that the person you are talking with understands what you have said, staff may ask, "Many people have a hard time understanding information like this. I want to make sure that I explain it clearly. Could you please tell me, in your own words, what I have just said?" When translating materials, at least two bilingual, bicultural translators should be used. In addition, materials should be pilot-tested with the target audience.
Program strategies:
Take the outreach program out to community and
neighborhood centers as well as to other sites that are already
familiar to Hispanics. Consider partnering with existing groups. Use family-oriented, positive messages as much as
possible to offer hope. Use radio and newspapers for outreach. Spanish-language
media-especially television and radio talk shows-are popular sources
of health-related information for many in these communities.
Printed materials with many pictures/illustrations and minimal text are preferred.
Older Adults
When considering older people and clinical trials, it is important
to note that approximately 60 percent of all cancers occur in people
aged 65 and older, and the number of people over age 65 is expected
to double by the year 2033. The elderly are an important group to
participate in clinical trials.
Because of mobility problems, transportation (including escort
assistance) is one of the most important challenges specific to older
people. Literacy issues (see below) are also a challenge that must be
addressed when educating older adults.
Fostering positive doctor-patient interaction is another
difficulty because often the older population is reluctant to
question or challenge doctors and may be afraid to offend by changing
doctors. This is compounded when doctors do not refer older adults to
trials because of the assumption that they are too old or sick for a
trial.
Older persons are more likely to be living on fixed incomes, so
the financial aspects of clinical trial participation may be
heightened.
The family, or other social support, is another important
consideration because it is often involved in the older person's
treatment and decision-making process.
It is important to inform older adults that Medicare reimburses
for all routine care costs for its beneficiaries participating in
clinical trials.
People with Low Literacy Skills19
"Many Americans face the serious problem of not being able to read
or understand information. According to the 1992 National Adult
Literacy Survey (NALS), some 40 to 44 million of the 191 million
adults in the United States are functionally illiterate. Another 50
million are only marginally literate. Functional literacy represents
more than just the ability to read. It involves reading comprehension
as well as the ability to compute, communicate, write, and solve
problems. These skills are especially important for patients in
acquiring general information and applying it to their specific
circumstances."
"When applied to the health system, low functional literacy
translates into low health literacy. Health literacy is defined as
the ability to obtain, interpret, and understand basic health
information and services, as well as competence and motivation to use
such information and services in ways that enhance one's health. Most
health-related educational materials use simplified printed materials
to convey information, assuming that people can read. Most adults do
read, but many have difficulty understanding what they read and
applying generalized information to their own specific
situation."
"One common assumption is that certain populations have low levels
of functional literacy. For example, traditionally "underserved"
populations such as those with low incomes are labeled as having low
levels of functional literacy simply because they are, on average,
less educated. However, low functional literacy is not defined by
race, class, or even educational attainment."
Ways to Help People with Low Health Literacy Skills
"One-on-one assistance is the most effective technique for
educating this group. In addition to helping people gain a better
understanding of the clinical trial and their health needs,
one-on-one assistance fosters trust between patients and the
counselors or health care professionals who help them. Comprehension
should be ascertained, but not by asking, "Do you understand?" Often
the "teach back" method works well."
"Group assistance offers an arena in which people can
obtain information from educators and through the questions asked by
others in the group. This technique often supplements one-on-one
counseling."
"Visual tools are designed to simplify concepts such as
instructions for care that are too complicated to understand in
written form or through verbal communication. Visual tools are
particularly useful to those who cannot read at all. Videotapes may
be useful tools, but follow up discussion is necessary in order to
ascertain comprehension."
Lesbian, Gay, and Bisexual Individuals
The lesbian, gay, and bisexual (LGB) community is diverse in terms
of cultural background, ethnic or racial identity, age, education,
income, rejection or acceptance of societal stereotypes, and
prejudice. As with other minority groups, discrimination and bias can
play a role in inadequate medical assessment, treatment, and
prevention of LGB health problems. In addition, lesbians may be at a
greater risk of cancer because of issues associated with health care
access, delayed or lack of childbearing, screening, and
insurance.
Little information is available about specific clinical trial
barriers for the LGB population. Significant barriers that must be
addressed include:
Previous negative health care experiences Fear of sexual orientation disclosure Perceived or actual exclusion from health promotion
campaigns Misinformation about risks and screening Exclusion of significant others
1. U.S. Census Bureau. U.S. Department of Commerce, Census 2000. Available at http://www.census.gov
1a. U.S. Census Bureau. U.S. Department of Commerce Health Insurance Coverage: 2001 Issued September 2002. Available at http://www.census.gov
2. Sateren, W. B., Trimble, E. L., Abrams, J., Brawley, O., Breen, N., Ford, L.,
McCabe, M., Kaplan, R., Smith, M., Ungerleider, R., Christian, M. C. (2002).
How sociodemographics, presence of oncology specialists, and hospital
cancer programs affect accrual to cancer treatment trials. Journal of Clinical
Oncology Apr 15;20(8):2109-17.
3. Edwards, B.K., Howe, H.L., Ries, L.A.G., Thun, M.J., Rosenberg, H.M., Yanick, R., Wingo, P.A., Jemal, A., Feigal, E.G. (2002). Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on the U.S. cancer burden. Cancer May 15;94(10):2766-2792.
http://seer.cancer.gov
4. American Cancer Society. (2002). Cancer facts and figures
2002.
5. Roberson, N. L. (1994). Clinical trial participation:
Viewpoints from racial/ethnic groups. CANCER Supplement, 74(9),
2687-2691.
6. Guilano, A. R., Mokuau, N., Hughes, C., Tortolero-Luna, G.,
Risendal, B., Ho, R. C. S., Prewitt, T. E., & McCaskill-Stevens,
W. J. (2000). Participation of minorities in cancer research: The
influences of structural, cultural, and linguistic factors.
Annals of Epidemiology, 10(8), S22-S34.
7. Brown, D.R., Fouad, M. N., Basen-Enguist, K., &
Tortolero-Luna, G. (2000). Recruitment and retention of minority
women in cancer screening, prevention, and treatment trials.
Annals of Epidemiology, 10(8), S13-S21.
8. Royal, C. D., Baffoe-Bonnie, A., Kittles, R., Powell, I.,
Bennett, J., Hoke, G., Pettaway, C., Weinrich, S., Vijayakumar, S.,
Ahaghotu, C., Mason, T., Johnson, E., Obeikwe, M., Simpson, C.,
Mejia, R., Boykin, W., Roberson, P., Frost, J., Faison-Smith, L.,
Meegan, C., Foster, N., Furbert-Harris, P., Carpten, J.,
Bailey-Wilson, J., Trent, J., Berg, K., Dunston, G., & Collins,
F. (2000). Recruitment experiences in the first phase of the
African American hereditary prostate cancer (AAHPC) study. Annals
of Epidemiology, 10(8), S68-S77.
9. Lee, M. (1999). Breast and cervical cancer early detection
in Chinese American women. Asian-American Pacific Islander
Journal of Health, 6, 358-367.
10. Taylor, V., Jackson, J., Schwartz, S., Yasui, Y., Tu, S.,
& Thompson, B. (1999). Cervical cancer control in a Cambodian
American population. Asian-American Pacific Islander Journal of
Health, 6, 368-377.
11. Shinagawa, S. M., Kagawa-Singer, M., Chen, M. S., Tsark, J.
U., Palafox, N. A., & Mackura, G. (1999). Cancer registries
and data for Asian Americans and Native Hawaiians and Pacific
Islanders: What registrars need to know. Journal of Registry
Management, 26(4), 128-141.
12. Miller, B.A., Kolonel, L.N., Bernstein, L., Young, Jr., J.L., Swanson, G.M., West, D., Key, C.R., Liff, J.M., Glover, C.S., Alexander, G.A., et al. (Eds). (1996). Racial/ethnic patterns of cancer in the United States 1988-1992 (NIH Publication No. 96-4104). Bethesda, MD: National Cancer Institute.
13. Papa Ola Lokahi. (May 1, 2001). Personal communication.
Honolulu, HI and Tsark, J.U. (1998). Cancer in Native Hawaiians.
Pacific Health Dialog, 5, 315=327.
14. Hodge, F. S., Weinmann, S., & Roubideaux, Y. (2000).
Recruitment of American Indians and Alaska Natives into clinical
trials. Annals of Epidemiology, 10(8), S41-S48.
15. Burhansstipanov, L. (Director, Native American Cancer Research
Corporation). (April 2001). Interview. Pine, CO.
16. U.S. Department of Health and Human Services. (1997). Regional differences in Indian health. Rockville, MD: Indian Health Service.
17. Lanier, A. P., Holck, P., Kelly, J., Smith, B., & McEvoy,
T. (1999). Alaska Native cancer survival report. Anchorage,
AK: Alaska Native Health Board.
18. U.S. Census Bureau. US Department of Commerce, Health Insurance Coverage, by Selected Characteristics: Issued October 1999. Available at http://www.census.gov
19. American College of Physicians-American Society of Internal
Medicine. (2000). No health insurance? It's enough to make you
sick. Latino community at great risk. Philadelphia: Author.
Available: American College of Physicians-American Society of
Internal Medicine, 190 N. Independence Mall West, Philadelphia, PA,
19106.
20. National Cancer Institute. (1996). Communicating with
Hispanic cancer patients: A focus group study. Washington, DC:
Author.
21. Center for Medicare Education. (2000). Considering health literacy. (Issue brief, volume 1, no. 6. [Online], Available: http://www.MedicareEd.org
Other sources utlized for this section include:
Alexander, G. A., Chu, K. C., & Ho., R. C. S. (2000).
Representation of Asian Americans in clinical cancer trials.
Annals of Epidemiology, 10(8), S61-S67.
Atkinson, J., & Hartmuller, V. (1994). Strategies for
minority recruitment. In PCPT minority recruitment manual.
Bethesda, MD: National Cancer Institute.
Brant, J. (1996). Breast cancer challenges in American Indian
women. In K. H. Dow (Ed.), Contemporary issues in breast cancer
(pp. 243-252). Sudbury, MA: Jones and Bartlett.
Brant, J., Fallsdown, D., & Iverson, M. (1999). The
evolution of a breast health program for Plains Indian women.
Oncology Nursing Forum, 26(4), 731-739.
Bunn, P., & Krebs, L. (1997). Colorado blueprint: Women and
minorities in cancer care trials. Denver, CO: University of
Colorado Comprehensive Cancer Center.
Center for Lesbian, Gay, Bisexual, and Transgender Health. (2000).
Lesbian, gay, bisexual, and transgender health: Findings and
concerns. New York: Columbia University's Joseph L. Mailman
School of Public Health.
Chen, A. M. (1996). Demographic characteristics of Asian and
Pacific Islander Americans: Health implications. Asian-American
and Pacific Islander Journal of Health, 4, 40-49.
Chu, K. C. (1998). Cancer data for Asian Americans and Pacific
Islanders. Asian-American and Pacific Islander Journal of Health,
6(2), 130-139.
Haynes, M. A., & Smedley, B. D. Committee on Cancer Research
Among Minorities and the Medically Underserved. (1999). The
unequal burden of cancer: An assessment of NIH research and programs
for ethnic minorities and the medically underserved. Health
Sciences Policy Program, Health Sciences Section, Institute of
Medicine. Washington, D.C.: National Academy Press.
Hughes, C. K, Tsark, J. T., Kenui, C. K., & Alexander, G. A.
(2000). Cancer research studies in Native Hawaiians and Pacific
Islanders. Annals of Epidemiology, 10(8), S49-S60.
Kagawa-Singer, M., Millon-Underwood, S., Burhansstipanov, L.,
& Munet-Vilaro, F. (1994). Nursing research and underserved
populations. In Proceedings of the Third National Conference on
Cancer Nursing Research. Atlanta, GA: American Cancer Society.
Lin-Fu, J. S. (1994). Ethnocultural barriers to health
care: A major problem for Asian and Pacific Islander Americans.
Asian-American and Pacific Islander Journal of Health, 2,
290-298.
McCabe, M. S., Varricchio, C. G., & Padberg, R. M. (1994).
Efforts to recruit the economically disadvantaged to national
clinical trials. Seminars in Oncology Nursing, 10(2),
123-129.
Millon-Underwood, S. (1994). Barriers to minority participation
in clinical trials. In PCPT minority recruitment manual.
Bethesda, MD: National Cancer Institute.
Millon-Underwood, S., Sanders, E., & Davis, M. (1993).
Determinants of participation in state-of-the-art cancer
prevention, early detection/screening, and treatment trials among
African-Americans. Cancer Nursing, 16(1), 24-33.
National Cancer Institute. (1997). Talking to patients and the
public about cancer clinical trials: Findings from NCI's "Science
Awareness Research." Bethesda, MD: Author.
National Clearinghouse for Alcohol and Drug Information. (2000).
[Online], Available:
http://www.health.org/ 2
Paskett, E. D., DeGraffinreid, C., Tatum, C. M., & Margitic,
S. E. (1996). The recruitment of African-Americans to cancer
prevention and control studies. Preventive Medicine, 25,
547-553.
Shinagawa, S. M., Kagawa-Singer, M., Chen, M., Tsark, J., Palafox,
N., & Mackura, G. (1999). Cancer registries and data for
"Asian Americans" and "Native Hawaiians and Pacific Islanders": What
registrars need to know. Journal of Registry Management, 26(4),
128-141.
Susan G. Komen Breast Cancer Foundation. (1999). Suggestions to
enhance the participation of women of color in breast cancer
prevention trials. In Clinical Trial Community Outreach Efforts.
Dallas, TX: Author.
The Mautner Project for Lesbians with Cancer. (2001).
[Online], Available: http://www.mautnerproject.org.
Thomas, S. B., Quinn, S. C., Billingsley, A., & Caldwell, C.
(1994). The characteristics of Northern Black churches with
community health outreach programs. American Journal of Public
Health, 84(4), 575-579.
Underwood, S. (2000). Minorities, women and clinical cancer
research: The charge, promise, and challenge. Annals of
Epidemiology, 10(8), S3-S12.
Ways to Educate Yourself
What You Can Do
Use NCI resources to keep up-to-date with new
clinical trial information, including www.cancer.gov Find local clinical trials in your community Attend conferences, meetings, and workshops
Why?
Introduction
One of the most important ways to get started in clinical trial
outreach and education is to make sure you learn as much as you can
about clinical trials. This section describes ways you can learn more
about clinical trials.
Education and Outreach in Action
|
A 60-year-old insurance salesman was treated for prostate
cancer 2 years ago. He recently decided that he wanted to learn
more about clinical trials after he saw a TV special about them.
"I'm amazed about what I don't know about what clinical trials are
going on right here in (my community). I want to make sure that
men who go to my support group understand information about
clinical trials."
|
The NCI Web site, www.cancer.gov,
contains information from PDQ®, including the latest information
about cancer treatment, screening, prevention, genetics, supportive
care, and complementary and alternative medicine, as well as a
registry of cancer clinical trials. Clinical oncology specialists
review current literature from more than 70 medical journals,
evaluate its relevance, and synthesize it into clear summaries, which
are then reviewed monthly and updated as needed based on new
information. Most cancer information summaries appear in two
versions: a technical version for the health professional and a
nontechnical version for patients, their families, and the public.
Many of the summaries are also available in Spanish.
The NCI Web site also includes approximately 100 fact sheets on
various cancer-related topics and information on ordering NCI
publications, as well as educational features and news summaries
concerning the latest results from cancer clinical trials.
The clinical trials registry (PDQ) contains more than 1,800
ongoing clinical trials, with information about studies around the
world. All clinical trials undergo review prior to inclusion.
Although no single resource lists every cancer clinical trial being
conducted in the United States and abroad, PDQ is the most
comprehensive cancer clinical trials registry, and contains
information about trials sponsored by NCI, the pharmaceutical
industry, and some international groups. Users can narrow their
retrieval by multiple parameters, such as stage of disease, phase of
trial, treatment modality, and geographic location. PDQ also contains
an archival file of more than 11,000 clinical trials that are no
longer accepting patients, including contact information for the
principal investigators of trials that may not yet be published in
the biomedical literature.
Accessing Information from NCI
NCI's Cancer Information Service
NCI's Cancer Information Service is a national information and
education network for patients, the public, and health professionals.
From regional offices covering the entire United States, Puerto Rico,
and the U.S. Virgin Islands, trained staff provide the latest cancer
information through a toll-free telephone service. Staff can respond
to calls in either English or Spanish.
How to Reach the CIS
The toll-free number is 1-800-4-CANCER (1-800-422-6237). For deaf
and hard of hearing callers with TTY equipment, the number is
1-800-332-8615. Hours of operation are Monday through Friday, 9:00
a.m. to 4:30 p.m., local time. Callers also have the option of
listening to recorded information about cancer 24 hours a day, 7
days a week.
See the Guide to Finding Clinical Trial Resources for other sources of clinical trial information.
A good way to educate yourself about clinical trials is to become
familiar with both the local institutions conducting clinical trials
and the clinical trials themselves. As you begin to think about ways
you would like to take action, it will be important for you to
understand where clinical trials are taking place.
Creating a List
You may want to create a list that identifies cancer clinical
trials in your community. Collecting this information using the
"Clinical Trial Community Resource Sheet" here can help you
direct interested community members to the best clinical trial
resources available.
This information can help you:
Understand clinical research in your community Understand some of the barriers to participating in
clinical trials in your community Provide local clinical trial resources to the organizations
or individuals with whom you are working
Clinical Trial Community Resource Sheet
You may use this sheet as a reference for yourself or your
organization, or you may develop it into a local fact sheet for
distribution. Remember that clinical trial information becomes out of
date very quickly, so you will need to update this sheet at least
every 3 months. To locate information needed to complete this
worksheet, you can:
Call the National Cancer Institute's (NCI's)
Cancer Information Service at 1-800-4-CANCER. Trained information
specialists can search the NCI clinical trial database, PDQ, and
provide contact information for local trials. Visit the Web site www.cancer.gov, to search the PDQ
database on your own. Use the clinical trial resources sheet that follows for
other ways to find local trials.
|
Type of Trial/ Type
of Cancer
|
Phase
|
Protocol ID#
|
Institution
|
Point of Contact
|
Phone
Number
|
|
filler
|
filler
|
filler
|
filler
|
filler
|
filler
|
|
filler
|
filler
|
filler
|
filler
|
filler
|
filler
|
|
filler
|
filler
|
filler
|
filler
|
filler
|
filler
|
|
filler
|
filler
|
filler
|
filler
|
filler
|
filler
|
|
Guide To Finding
Clinical Trial Resources
|
|
National Cancer Institute's PDQ 3
|
What is it?
|
How do I access it?
|
What will it provide?
|
|
Database produced by NCI
Registry of approximately 1,800 active cancer clinical
trials
|
Go to http://cancer.gov/clinicaltrials/
Use the search form
OR
Call 1-800-4-CANCER
|
Summaries about clinical trials conducted by
NCI-sponsored researchers, the pharmaceutical industry, and
some international groups
|
|
National Library of
Medicine
|
Database produced by NIH Registry now lists 4,000
primarily NIH-supported clinical studies on many conditions,
and more will be added
All trials on PDQ are listed in this database
|
Go to www.clinicaltrials.gov
Can browse by disease or sponsor or insert key words
|
Summaries about clinical trials for a wide range of
conditions-most of the trials listed are sponsored by
NIH
|
|
Local Cancer Center Web Sites
|
Locally produced Web sites that include listings for
trials sponsored by NCI and some pharmaceutical
companies
Good supplementary resources for locating clinical
trials; a cancer center may begin participating in an
NCI-sponsored trial before the center's information is
listed in PDQ
|
Different sites can be found through:
• PDQ 3 and National Library of Medicine 4 Web
sites
• Local institutions
Information on trials taking place at NCI's Clinical
Center in Bethesda, Maryland, is available at http://ccr.nci.nih.gov
Some centers may also have telephone information
centers
|
Information that varies from center to center
|
|
Examples of Pharmaceutical Resources/Internet Clinical
Trial Matching Sites
|
Pharmaceutical Research and Manufacturers of America
(PhRMA) publishes a list of new cancer drugs in
development
CenterWatch's Clinical Trials Listing Service lists many
industry- and Government-sponsored trials
|
PhRMA
Go to www.phrma.org/
Click on "New Medicines in Development."
The drugs are listed by cancer type or call 202-835-3400.
CenterWatch
Go to www.centerwatch.com
Click on "Trial Listings"
EmergingMed.com
Go to www.emergingmed.com or call 877-601-8601
|
Descriptions, sites, telephone numbers and investigator
names by State
|
Some people find that attending scientific meetings keeps them up
to date, helping them become better educators.
Local Meetings and Courses
Many local hospitals and cancer centers hold public events,
educational workshops, and lectures on a variety of topics related to
clinical trials. Contact your local institution's community relations
department to find out if it holds such events and when they are
scheduled.
Learning through the Internet
Many of the large cancer centers have free online courses for the
public, some of which may be related to clinical trials. See the Web
site www.cancer.gov for a list of NCI-designated cancer centers and
their Web sites to learn more about specific online courses.
National Meetings
Scientists present the results of their research at large meetings
before the results are published in the literature. Two annual
meetings of importance to cancer research are:
American Association of Cancer Research in March of each year
(Web site is www.aacr.org) American Society of Clinical Oncology in May of each year (Web
site is www.asco.org)
Both feature daily meeting updates on their Web sites.
Many scientific meetings are open and free to the public. Others
have reduced fees for survivors or advocates.
The NCI event calendar provides a centralized and easily
accessible place to obtain information about many cancer-related
scientific meetings and events nationwide. The calendar can be found
on the Web at www.cancer.gov. You
can also call 1-800-4-CANCER to get information about these
meetings.
Ways to Educate Others, One-on-One
What You Can Do
Find opportunities to talk to others-neighbors,
friends, and coworkers-about clinical trials, common myths
surrounding clinical trials, and where to find more information on
clinical trials Help others understand how to evaluate research in the
news
Why?
Introduction
As someone who knows a lot about clinical trials, you will often
be given the opportunity to talk to individuals about their cancer,
cancer of a loved one, or being at high risk for cancer. One of the
best ways to educate other people is to talk informally about what
you are learning about clinical trials.
As you seek opportunities to speak to others, keep in mind that
it's important to be able to answer common questions about clinical
trials. But it's also important to admit when you don't know the
answers, and to seek other resources for information.
This section will guide you through some ideas to talk to
others
Education and Outreach in Action
|
A high school teacher who is a cancer survivor
is finding ways to spread the word about clinical trials.
Because she is active in her church, she finds herself
talking to many people whose lives have been touched by
cancer. She says, "People call me the cancer lady, but I
don't care." She is often asked to talk to fellow church
members about her experience with cancer and her
treatment on a clinical trial.
She says, "I used to think that clinical trials
treated people like guinea pigs-a lot of folks do. I
think people need to know the facts about clinical
trials. No one likes to hear their own name associated
with the word 'cancer.' But, how many times does someone
hear about a co-worker, a friend, or a neighbor who was
just diagnosed with cancer? That's how I approach other
people. I tell them that it's important that cancer
patients talk to their doctor about all their options.
That's the best help that I can provide someone."
|
Finding Opportunities to Talk to Others
You may be active within one specific organization or have
connections with many different community groups. As you think about
the importance of clinical trials, think about where and how you can
become a clinical trial resource to people you know. How can you help
other people learn about clinical trials?
In the circles below, write down the names of at least four people
with whom you feel you can discuss clinical trials. Think about these
people as you work through this section.
One way to provide support to others is to help those diagnosed
with cancer, and those at high risk for developing cancer, to learn
more about the option of participating in a clinical trial. Equally
important is explaining clinical trials to those who are not facing a
cancer diagnosis, but who are interested in why they are
important.
Here are some tips to get you started on this conversation; keep
in mind that conversations need only be a few minutes long:
Express empathy for the person's situation. Use active listening skills. Ask whether the person would like to hear some information about
cancer clinical trials. In your own words, talk about why clinical trials are
important.
If relevant:
Discuss clinical trials as an option for treatment and
prevention. Remember that clinical trials are not the right option
for everyone. Review some of the benefits and risks to participating in clinical
trials. Respond to common myths associated with clinical trials. Explain some key ways that participants' safety is protected in
clinical trials. Explain some of the barriers associated with clinical trials for
patients. Know how to answer common questions about clinical trials. But,
admit when you don't know the answers. Have resources available (such
as those in this series) and the Cancer Information Service's
1-800-4-CANCER. Be aware of cultural sensitivities around clinical trial issues.
(See General Strategies for Educating Diverse Populations. 6) Understand the local clinical trial resources in your
community.
People often hear about the results of clinical trials from overly
positive or overly negative media reports. These reports often
influence the way they think about clinical trials and can help to
reinforce common myths. When you find yourself talking to others
about clinical trials, it will be important for you to respond to
these reports in a thoughtful way. Here are some questions to
consider as you help others evaluate these types of media
reports.
Some of these questions will not be answered by a short article in
a newspaper. You may need to locate the original research article by
using PubMed or another medical library database.
Who wrote this article?
Any good news article should list the author,
his or her institution, and if the author is not a
reporter, his or her background. Reporters are trained to
be objective, but they often report on what seems
newsworthy and may miss key facts. Some institutions may
not list the author of an article, in which case the
institution itself should be considered the author.
Newspapers often use "wire reports," such as the
Associated Press (AP), and often include materials
excerpted directly from press releases. What is the basis of the information in the
article?
In addition to identifying who wrote the article
you are reading, the evidence that material is based on
should be provided. Medical facts and figures should have
references (such as an article in a medical journal or
the consensus of a meeting of experts reviewing research
evidence); anecdotal evidence, opinions, or advice should
be clearly set apart from information that is
"evidence-based" (that is, based on research results). Does the article say if the study involved people,
animals, or cells in the laboratory?
In early cancer research or preclinical testing,
scientists test promising new cancer treatments in the
laboratory and in animal models. This is done to find out
if the treatment has an anticancer effect and if it is
safely tolerated in animals. Only if a new drug proves
promising in the lab, does it move to testing in humans.
Clearly, preclincial research only suggests future
direction, and cannot be applied directly to all people
with a particular type of cancer. Does the article include information on the phases of the study
and number of participants?
Clinical research is generally conducted in
three different types of studies called phase 1, 2, and 3
studies, reflecting the order in which they take place.
Each phase of a study has a different goal and, although
they are often conducted separately they can be conducted
as part of the same study.
News articles may not mention the study phase.
However, if an article refers to an "early" study, it
generally refers to a phase 1 or 2 study. If an article
discusses different groups of patients getting different
types of treatment or different doses of a drug, or
mentions that a study was "randomized," it usually means
the study was a phase 3 study. Occasionally, phase 2
studies may be randomized. If the study includes a new agent or device, has it
been approved by FDA?
The Food and Drug Administration (FDA), an
agency of the U.S. Department of Health and Human
Services, must review all test results for new agents to
ensure that products are safe and effective for specific
uses.
FDA applies two key questions to each application for
drug approval:
Do the results of well-controlled studies
provide substantial evidence of effectiveness?
Do the results show the product is safe under
the conditions of use in the proposed labeling? (In this
context, "safe" means that potential benefits have been
determined to outweigh any risks.)
Only when FDA makes the approval does it allow the
drug to be "labeled" for a specific use. This label
includes information on what kinds of people should be
given the drug, the dosage of the drug, and information
on safety and adverse effects. It is only at this time
that the new drug can be given to people outside of a
clinical trial. What were the characteristics of the people who
participated in the study?
Sometimes, studies are done only in specific
groups of people-for example, people in a particular age
group, or those whose cancer is at a particular stage.
This may mean that the study results may not apply to
those who are not members of these specific groups. Does the article discuss the benefits and risks for
those participating in the study in a balanced way?
Just as there are potential side effects to all medications, there are benefits and risks to all clinical trials. The article should fully describe the known benefits and risks, as well as note that there may be side effects or risks that are unknown. If the article appears to be one-sided or overtly positive, you may be justified in having some skepticism about the author's motives. Who funded the study?
Studies can be funded from many sources. The
most common sources in the United States are the Federal
Government, universities, private foundations, or
industry (such as pharmaceutical companies or companies
making a device that is being tested). If a researcher
has received funding from a source, such as the Federal
Government, that uses a peer review process for
evaluating and awarding research funds, the research
findings will be more credible and less subject to bias
than if the sponsor has a vested interest in the results. Who conducted the study?
It is important that research studies have
scientific review and ongoing oversight. Studies that
have been through this level of scrutiny are more likely
to produce high quality results than those that have not
been reviewed. Were the study results "peer reviewed" or just
announced at a meeting or through a press release?
If the news article was based on a published
article in a peer-reviewed journal, it means that other
experts have examined and critiqued the researchers'
methods and findings. |
Ways to Conduct Community Outreach and Education
What You Can Do
Find opportunities and develop a strategy to speak about
clinical trials and/or:
Present NCI Clinical Trials Education Series slide programs or the awareness video
"Cancer Trials…Because Lives Depend on It" Distribute educational booklets and brochures from the
Clinical Trials Education Series: 1
Write articles for newsletters; post articles on Web
sites Host an information session or community forum on clinical
trials Form a clinical trial advocacy coalition
Why?
To provide information to people about cancer clinical
trials before they are faced with a cancer diagnosis To provide information about a specific cancer trial to
members of your community To provide people with the tools they need to find out
about trials in your community To educate interested members of the public about the
importance of cancer clinical trials To help debunk myths about clinical trials in the
community To develop a community strategy for clinical trial
outreach
Introduction
This section provides ideas for educating your community about
clinical trials. Whether you use personal contacts, community
organizations, or health-related organizations, remember that
combining strategies to get clinical trial messages out to your
community will make your outreach more successful. Using different
ways to get out critical messages about clinical trials increases the
chance that people in your community will absorb and remember
them.
Education and Outreach in Action
|
A chapter of a local cancer advocacy
organization had been trained about clinical trials.
Several members organized a community forum, inviting the
local researchers on a breast cancer prevention trial to
speak. They also invited several women who considered
participating in the trial. More than 75 people attended
the forum at the local YMCA.
|
|
A local cancer support group has a Web site and
a quarterly newsletter. The group's education committee
copied the articles included here and put them on its Web
site and in its newsletter. At the next meeting, the
group decided that all of its members should learn about
clinical trials.
|
Everyone has connections with different groups in his or her
community. Some people represent a specific organization. Others may
have connections with many different community groups.
As you think about the importance of clinical trials, think about
where and how you can become a clinical trial resource to these
groups. How can you help engage the organization in this issue?
In the circles below, write down at least five groups or
organizations you feel you can educate. The groups don't need to be
cancer-related, but should have a health focus. Keep these groups in
mind as you complete this section.

It is important to think broadly about other organizations with
which you can partner, whether you are part of a group or
organization that already has a clinical trial agenda, or if you want
your group to develop one.
A given geographical area includes many "communities." In the
following table, write down the organizations you identified on the
previous page.
Then, think about:
Reaching out to other groups in your community who are
likely to share clinical trial priorities Partnering with organizations with which you haven't yet
worked Contacting people who can put you in touch with key
leaders of these groups
The pages that follow will help you prioritize your outreach
efforts. It will be important to consider balancing your efforts
between groups that are easy to reach with others that will take more
effort.
Organization Contact Worksheet
|
Organization Type
|
Examples in My Community
|
Contact People in Community
|
|
Advocacy organizations
|
filler
|
filler
|
|
African American, Asian American, Latino, and Native
American-based organizations
|
filler
|
filler
|
|
Cancer-oriented nonprofit organizations
|
filler
|
filler
|
|
Cancer support groups
|
filler
|
filler
|
|
CDC Breast and Cervical Cancer Early Detection Program
coalition members (hint: check with your State health
department)
|
filler
|
filler
|
|
Chambers of commerce
|
filler
|
filler
|
|
Community cancer centers
|
filler
|
filler
|
|
Community health centers/public health clinics
|
filler
|
filler
|
|
Employee associations of large companies
|
filler
|
filler
|
|
Health care professional associations (doctors, nurses,
social workers, health educators, etc.)
|
filler
|
filler
|
|
Hospital education departments
|
filler
|
filler
|
|
Hospitals and research institutions
|
filler
|
filler
|
|
Housing organizations
|
filler
|
filler
|
|
Labor union locals
|
filler
|
filler
|
|
Lesbian/gay organizations
|
filler
|
filler
|
|
Men's organizations
|
filler
|
filler
|
|
Religious organizations/houses of worship
|
filler
|
filler
|
|
Senior citizens' organizations
|
filler
|
filler
|
|
Service organizations, such as Rotary, Lions, Kiwanis,
Jaycees, Junior League
|
filler
|
filler
|
|
State cancer control committees
|
filler
|
filler
|
|
Veterans' groups
|
filler
|
filler
|
|
Women's organizations
|
filler
|
filler
|
As you begin to consider different outreach and education
activities, you'll need to think strategically. Answering the
following questions can help ensure the success of your outreach and
education efforts.
Setting Objectives
Why should we get involved with clinical trial outreach and
education? What is the problem we want to address? (Be realistic!) What is the goal or expected outcome of our efforts?
Conducting Research
Is our clinical trial advocacy goal clear and realistically
attainable? Who are our primary allies? Have similar clinical trial education efforts been
attempted in the past, either in our community or elsewhere? What
lessons can be learned from them? What is the most compelling information that we can use to
make our case? (Think about your targets as you answer this
question.)
Considering "Targets"
In figuring out who your audience is, keep in mind that the
"general public" is not specific enough to be a target audience.
Ask:
Who are the key targets for our efforts? Are there key people who can help our efforts in reaching
these audiences? Leaders of professional organizations? Community
leaders? Who is our "community," and how can it be expanded? (Use
your worksheet.)
Considering a Philosophy
The questions that follow represent a simplified framework for
outreach and education efforts that could be large and complex. The
more complete your initial answers, the more likely it is that your
efforts will proceed smoothly and successfully. Consider the
following:
What are the most appropriate ways for our community to
learn about clinical trials? Do we want to simply inform people? Or do we want to
influence attitudes and change behavior? Which channels best fit our message about clinical
trials? What avenues are most likely to be credible to and
accessible by our community or our "targets"? Meetings? Trainings?
The media? Mail? Networks of contacts? Doctors' offices? Community
races? How can we complement existing cancer awareness activities,
such as national theme months (e.g., October is Breast Cancer
Awareness Month), local health fairs, and local hospital events, in
our efforts? Who are our best spokespeople? How can we use community
leaders in our outreach efforts? Does timing play an important role in some of our planned
activities? Will other pending issues claim our targets'
attention?
|
If you are not from the population(s) you seek to work
with, it is critical that you or your organization develop
partnerships with organizations from those communities.
Keep in mind that different communities find different
information sources credible and may respond better to
certain outreach strategies. Forming partnerships with other
organizations, rather than simply asking them to join
existing efforts, can help ensure that your educational and
outreach efforts will be successful.
|
You can present clear information about clinical trials to local
groups by using one of NCI's three slide programs in the Clinical Trials Education Series 1 or the awareness
video, "Cancer Trials…Because Lives Depend on It." Most of these
materials are geared to the general public and feature interactive
discussion guides or talking points. By showing these materials at a
meeting or as part of a larger program, you can generate discussion
on the importance of clinical trials in your community.
Distributing materials is more than putting brochures out on a
display table. Consider the following ideas.
At Events or Meetings
At meetings in which everyone receives a packet of
material, target educational materials by inserting a cover letter
signed by an influential person in your community. At events, such as health fairs or lectures, place
materials out on a table. Offer candy or water and cups. Instead of
standing behind a table, think about standing in front, to make your
table look more inviting. Greet people warmly.
Through Doctors' Offices
Partnerships may be developed with health care providers to
educate their patients about clinical trials. Primary care providers
may be willing to discuss prevention trials with their healthy
patients. Some may be willing to send out materials to their
patients.
Through a Booth at Health Fairs or Events for Local Research Institution(s)
Often local institutions will be eager to host a booth to discuss
the clinical trials they offer. These booths can also enhance
community awareness around clinical trials.
Local organizations' Web sites, listservs, and newsletters can be
targeted and effective ways to reach community members. Use the
sample newsletter articles provided here to submit to organizational
newsletters and Web sites.
Your organizations' own publications are one likely avenue. Other
organizations may also be eager to publish information. Review each
organization's media to understand better what they are likely to
publish:
Who is the target audience? What types of articles are currently available? Who can help get this article published/online?
The Pros and Cons of Using Newsletters
Pros
Newsletters are tools for intra- or
interorganization communication. Newsletters help show how organized your group is and
help keep your members and colleagues current on your
activities. Newsletters have a very broad audience.
Cons
Newsletters take time, money, and skill to
produce. Newsletters are not well suited for publishing
late-breaking news.
Sample Newsletter Article 1
Newsletter
|
Clinical Trials: An Important Part of Our
Mission
[Insert how clinical trials relate to your
organization's mission, and why you are getting involved
with clinical trial outreach and education.]
Just as there are misconceptions about cancer screening
tests, there are misconceptions about clinical trials. When
people think about clinical trials, many imagine "guinea
pigs"-- participants getting inferior treatment or a sugar
pill (placebo) instead of actual medical treatment. Many
people also think that clinical trials are for only the
people who have no other treatment options available to
them. This article provides an overview of why clinical
trials are important, what they do, and why they are a
critical part of our work.
Why Clinical Trials Are Important
Clinical trials are a critical part of the research
process. Clinical trials help to translate basic scientific
research into new treatments. By evaluating the results of
these trials, researchers are finding better treatments for
cancer and ways to prevent cancer. The more people that
participate in clinical trials, the faster we can answer the
critical research questions that will lead us to better treatment
and prevention options for all cancers. Doctors will never
know the true effectiveness of a cancer treatment, or a way
to prevent cancer, unless they are able to involve more
people in clinical trials. And, research shows that few of
us really understand what clinical trials are.
Cancer Treatment Trials
Most cancer clinical trials are treatment studies. These
clinical trials involve people who have cancer. These
studies try to answer specific questions about and evaluate
the effectiveness of a new treatment or a new way of using
an old treatment. Treatment trials seek to find out:
- What new treatment approaches can help
people who have cancer?
- What is the most effective treatment for people
who have cancer?
In most cancer treatment trials, in which one treatment
is compared with another, people receive either the most
advanced and accepted treatment for the kind of cancer they
have-known as the "standard" treatment-or a new treatment
that has shown promise of being at least as beneficial as the
standard treatment, if not better. People in these trials do
not receive a placebo for their treatment.
In the past, clinical trials were sometimes seen as a
last resort for people who had no other treatment choices.
Today, many people with cancer, even those whose cancers
have not spread, get their first treatment in a clinical
trial. All people with cancer can benefit from learning
about all their treatment options, which include
participating in appropriate clinical trials.
Cancer Prevention Trials
Unlike treatment trials, cancer prevention clinical
trials are studies involving healthy people who are at high
risk for developing cancer. These studies try to answer
specific questions about and evaluate the effectiveness of
ways to reduce the risk of cancer. Prevention trials seek to
find out:
What approaches can prevent a specific type of cancer
from developing in people who have not previously had
cancer?
Risks and Benefits
Clinical trials have both benefits and risks--and they
are not the right option for everyone.
Possible Benefits
Possible Risks
New approaches may have side effects or
risks that are unknown. Even if a new treatment under study has
benefits, it may not work for every participant. Participants may have to pay for the costs of
travel, childcare, lost work hours, and meals.
[Discuss your community's education and outreach
efforts.]
How to Learn More about Clinical Trials
Call NCI's Cancer Information Service at
1-800-4-CANCER (1-800-422-6237) and ask for a customized search of the
PDQ database, which provides information on current studies.
Ask for the following pamphlets, which are available in
English and Spanish:
"Taking Part in Clinical Trials: What
Cancer Patients Need To Know"* "Taking Part in Clinical Trials: Cancer
Prevention Studies"* "If You Have Cancer... What You Should Know
about Clinical Trials"* (easy to read) "If You Have Cancer and Have Medicare...You Should Know About Clinical Trials"
Or you can visit the clinical trials area of the NCI Web
site at www.cancer.gov.
*Also available in Spanish
|
Sample Newsletter Article 2
Newsletter
|
Key Facts about Cancer Clinical Trials
Cancer clinical trials are research studies in which
people help doctors find ways to improve health and cancer
care. Each study tries to answer scientific questions and
find better ways to prevent, diagnose, or treat cancer.
These studies are the final step in the process of
developing new drugs and other means to fight disease.
Clinical trials are the way we make progress against
cancer.
Understanding that Cancer Affects All of Us
Cancer affects us all-whether we have it, care
about someone who does, or worry about getting it in the
future. Consider the impact of cancer in the United
States* in 2002:
Each year, about 555,550 people are expected
to die of cancer-more than 1,520 people a day. Cancer is the second leading cause of death, exceeded
only by heart disease. 1 of 4 deaths is from cancer. About 1,284,900 new cancer cases are expected to
be diagnosed each year. Research has shown that there are many differences
in who develops cancer and who dies from cancer among men
and women, and among people of different races, ethnicities,
and socioeconomic backgrounds. Differences in cancer
screening and treatment have also been documented for people
of different ages, incomes, educational, and racial/ethnic
backgrounds.
Understanding What Clinical Trials Do to Fight
Cancer
Clinical trials are a critical part of the
research process. Clinical trials help to translate basic
scientific research into practical treatments. By evaluating
the results of these trials, we can find better treatments
for cancer and ways to prevent cancer. Clinical trials contribute to knowledge and
progress against cancer. Many of today's most effective
cancer treatments are based on previous study results.
Because of progress made through clinical trials, many
people treated for cancer are now living longer. The more people that participate in clinical
trials, the faster we can answer the critical research
questions that will lead us to better treatment and
prevention options for all cancers. We will never know
the true effectiveness of a cancer treatment, or a way to
prevent cancer, unless we are able to involve more people in
clinical trials. In the past, clinical trials were sometimes seen
as a last resort for people who had no other treatment
choices. Today, many people with cancer, even those whose
cancers have not spread, get their first treatment in a
clinical trial.
Understanding How Few People with Cancer Take Part in
Clinical Trials
Enormous improvements in treating childhood
cancers have come about as the direct result of clinical
trials; more than 60 percent of U.S. children with cancer
participate in clinical trials. In 2000, more than 70
percent of children with cancer are alive 5 years after
diagnosis, compared with only 55 percent in the
mid-1970s. In contrast, only 3 percent of U.S. adults with
cancer participate in clinical trials-far fewer than the
number needed to answer the most pressing cancer questions
quickly. According to a survey in 2000, most people with cancer
were either unaware or unsure that participation in clinical
trials was an option for their treatment, and most of them
said they would have been willing to enroll had they known
it was possible.
How to Learn More about Clinical Trials
Call NCI's Cancer Information Service at
1-800-4-CANCER (1-800-422-6237) and ask for a customized search of the
PDQ database, which provides information on current studies.
Ask for the following pamphlets, which are available in
English and Spanish:
"Taking Part in Clinical Trials: What Cancer
Patients Need To Know"** "Taking Part in Clinical Trials: Cancer Prevention
Studies"** "If You Have Cancer... What You Should Know about
Clinical Trials"** (easy to read) "If You Have Cancer and Have Medicare...You Should Know About Clinical Trials"
Or you can visit the clinical trials area of the NCI Web
site at www.cancer.gov.
**Also available in Spanish
|
Many organizations choose to hold community events on clinical
trials, which can be an effective way to reach community members.
Careful planning is required to ensure your event is a success.
Step 1. Establish a Community Forum Organizing Committee, If You
Are Planning a Large Event
The organizing committee could include members from your education
committee or your board. You will need to determine a budget for the
project that includes costs of meeting room rental, printing flyers
or invitations, advertisements, and postage.
Involve other parts of your community-such as the leadership of
other nonprofit or advocacy organizations-in the planning
process.
The entire committee may need to meet only a few times to discuss
presentation goals and outreach strategies. A timeline and specific
"job responsibilities" can be discussed at the first meeting.
Step 2. To Focus Your Information Session, Clarify Your
Goals
It is important to answer the following question before beginning
to plan the event. Potential answers are provided here, but you
should answer this question for yourself: What is the goal in hosting
this forum?
You should aim for goals that are clearly expressible. Try not to
be overly ambitious for one community presentation. Getting community
leaders involved with clinical trials and educating community members
about clinical trials should be among your primary goals.
Specific goals of the meeting/forum might be to:
Provide information about a specific cancer trial
to members of your community. Develop a community strategy for clinical trial
outreach. Provide people with the tools they need to find out
about trials in your community. Educate the public about the importance of cancer
clinical trials. Help debunk myths about clinical trials. Make clinical trial information more accessible. Share information with local organizations, compare
strategies, and unify positions on common issues. Encourage people to inform others about clinical
trials. Begin to build links between local researchers with
community members.
Step 3. Determine Your Target Audience
Think about who and why. Develop a target audience list. Be sure
to involve community leaders.
Possible audiences include those listed in the section, "Expanding
Your Organization's Community." 9
|
For ideas in reaching cancer-related organizations in
your community, use the following sources from the National
Cancer Institute's Cancer Information Service:
|
Step 4. Choose an Interesting Theme with a Catchy Title
The theme should combine your organization's goals with what you
believe your target audience will find of interest. Some ideas are
given here.
General Information on Clinical Trials
What are clinical trials and why should we care about
them? How are cancer prevention clinical trials different from
other studies? What are the myths and facts about clinical trials? How are people protected in clinical trials? How does cancer research in our community address the
concerns of people living with the disease? How can someone decide whether to participate in a clinical
trial? What are the barriers to accessing clinical trials?
Specific Information on a Particular Trial
Provide information about a specific prevention or treatment trial
that may be having problems accruing patients. The presentation can
explain why it is important, including the risks and benefits. If
possible, give the locations of the trial sites in your area.
Community Advocacy for Clinical Trials
How can we help reduce the barriers to participation in
clinical trials in our community? How can we advocate for third-party payers to cover
clinical trial costs? How can we develop a community strategy for clinical trial
outreach? How can we make clinical trial information accessible to
those who need it in our community? How can we find ways to help people understand that
clinical trials can be a viable treatment option? How can people newly diagnosed with cancer learn about
clinical trials in the community?
Step 5. Choose the Forum Speaker(s), Format, and Date
Be sure to invite people who:
Can speak to lay audiences without using a lot of technical information Have a good rapport with the audience Live in the area Present information that people can use
|
Tip
When planning a special event, keep in mind that many
audiences are unfamiliar with scientific terms. Strive to
make information presented at your special event easy to
understand and creative.
|
For speaker ideas, contact principal investigators from the list
of cancer trials in your community. (For more
information, see Work with Researchers and Health Care Professionals. 11)
Consider inviting speakers who have actually participated in a trial. You can also contact your local cancer center,
hospital, or medical center or the Cancer Information Service for
help in finding speakers who will appeal to your audience.
Choose your date carefully. Make sure it is a time when most
people can attend and that it doesn't conflict with another large
community event or a holiday.
|
Tip
If your speakers are your big draw, you may want to offer
several dates to accommodate their schedules before the date
is confirmed.
|
Step 6. Decide on the Best Way to Inform the Target Audience
about the Event
Your message should reflect the theme you've chosen. The cost,
timing, and available person-power will affect the method you choose
to publicize your event. Consider the following methods:
Mailing invitations or flyers to organizational
mailing lists E-mailing event announcements to organizations and
individuals Posting on Web sites Advertising in local media, such as radio stations and
newspapers Distributing flyers or posters in the community Contacting cancer centers, hospitals, support groups,
and patient groups
Step 7. Obtain Educational Materials
At the event, provide participants with handouts on the benefits
of cancer research (e.g., handouts in this series). Encourage them to
share information with friends, relatives, and other people in their
lives.
Step 8. Arrange for a Location
Choose a location that is accessible by various modes of
transportation.
Step 9. Select an Event Format
Research has shown that adults strongly prefer interactive
delivery methods, which allow them to learn new information by:
Seeing Experiencing Discussing
Many scientists do not use these delivery methods, but you can
encourage their use to promote effective discussions in community
presentations. Remember that you want to advocate for people to take
action after they leave the forum.
Be creative in your format. Adults often gain the most out of
presentations by figuring out how they can apply the information in
their lives immediately. Possible formats include:
Single speaker format. An easy option is a
presentation that includes an expert speaking and answering
questions about cancer research. For example, a doctor, research
scientist, or outreach coordinator who is involved in a cancer
clinical trial could speak about the work he or she is doing in
the community. Panel discussion. A small group of experts (usually
between three and five) discuss a topic among themselves in front
of an audience. Panel participants don't make formal
presentations. They exchange ideas through conversation. Panels
can expose the audience to different points of view about a single
subject. Audience members ask questions or comment on the subject
after a panel discussion. Usually, a time limit is set for each
question and for the entire question-and-answer period. Cancer
trial panels might include:
Survivors who reflect the diversity of your
community who can discuss their decisions about trial
participation (survivors who are community leaders or otherwise
well known in your community might be of particular interest to
your target audience) Cancer researchers who can discuss their work Survivors and scientists who can discuss both the personal
decisions and the science behind trials Symposium. A small number of experts make short
presentations in succession. Presentations usually range from 5 to
15 minutes each. Open forum. Members of the audience may participate at
any time during the meeting. Colloquy. During a panel discussion, audience members
may be invited to comment or ask questions if a panel member or
the chair perceives a need to clarify points, raise an issue, or
ensure that a misperception doesn't stand. Any interruptions of
the panel discussion must focus on the point at hand. When the
matter has been resolved, the organized discussion among panel
members resumes. Buzz session. The audience is divided into groups of six
to eight people for discussion of questions posed by the leader.
One person from each group may be asked to summarize the group's
discussion and report to the entire audience. Audience reaction team. Three to five members of the
audience are pre-selected to respond to a presentation by offering
a brief summary and interpretation of information presented. This
discussion method can be used effectively in large-group settings
and when time is limited. Question period. Members of the audience have an
opportunity to ask questions of a presenter once the formal
presentation is completed. Usually, a time limit is set for each
question and for the entire question-and-answer period. Workshop. A small group of people (up to 25) with a
common interest meet to study, research, and discuss a specific
subject or to enhance their individual knowledge and
proficiency. Seminar. A group of people who are studying a specific
subject meet for a discussion led by a recognized authority.
|
Tip
No matter what format you choose, make sure you allow
plenty of time for questions and answers.
|
Working with Presenters
Preparation
Interview potential presenters in advance! You
need to assess a presenter's ability to discuss areas related to
clinical trials, and any personal experience he or she may have.
Ask potential presenters about their willingness to participate in
an event. Provide information about the date, time, and focus of
the event. Discuss any concerns about anonymity (e.g.,
picture-taking or media presence). Budget for honoraria and expenses. Whenever possible,
pay presenters an honorarium and offer food, mileage, parking, and
childcare reimbursement. If possible, plan to pay in cash the day
of the workshop (and get a signed receipt). Send a confirmation letter. Make sure it includes date,
time, and focus of the event, details about honorarium, travel to
the site, parking, and a telephone number of a contact person at
the training site. Call presenters 3 to 7 days before the event. Ask
whether they have questions. Discuss plans for focusing on
specific issues. Ask how they wish to be introduced. It may be
easiest for presenters to introduce themselves, so they can
disclose as much or as little identifying information as they
like. Obtain a facilitator/moderator. The facilitator's role
is critical to the event's success. A facilitator manages the
discussion. Presenters need support from the facilitator, who
should:
Assure them that they can refuse to respond to any
question for any reason
Monitor time carefully and gently, so everyone gets a
chance to speak
Help with questions and answers
Be available after the event to assist presenters and
offer feedback
Arrange to meet with all panelists before the
presentation. This will help alleviate their anxiety and provide a
chance to discuss any last-minute issues. Review the format for the panel. Look at time allowed
for each presentation and when/how questions will be taken from
the audience.
At the Event
Explain why you are holding a panel discussion. Hint:
Distribute an information sheet listing panelists and their
affiliations. Then you won't have to spend a lot of time on
introductions. Review the ground rules. Tell the audience how much time
each panelist will have to speak. Explain when audience members will
be able to ask questions or offer comments. Show courtesy and respect for differing points of view. Pay careful attention to respecting limits on time. Summarize in the last 5 minutes. This is important, even if
you have to say, "This discussion left many of us with more questions
than answers!"
Plan Your Community Events--A Sample Timeline
Whether you plan to host a forum or run a video presentation, this
checklist can help you plan.
6-8 Weeks in Advance
 Identify target audience Identify target audience
 Identify and reserve site for meeting and ensure video equipment
is available-will it be a part of a regular organizational meeting, a
visit to someone's home, a special event? Identify and reserve site for meeting and ensure video equipment
is available-will it be a part of a regular organizational meeting, a
visit to someone's home, a special event?
4 Weeks in Advance
 Refine target audience Refine target audience
 Review audience needs Review audience needs
 Develop and send e-mail/flyer Develop and send e-mail/flyer
 Order supplies, copies, and educational materials Order supplies, copies, and educational materials
 Confirm site reservation, video equipment Confirm site reservation, video equipment
2 Weeks in Advance
 Check in with onsite coordinator on number of participants and
any changes anticipated Check in with onsite coordinator on number of participants and
any changes anticipated
 Get flip charts and markers (if needed) Get flip charts and markers (if needed)
 Get nametags (if needed) Get nametags (if needed)
 Organize handouts Organize handouts
 Make sure any videotape works/CD-ROM works Make sure any videotape works/CD-ROM works
1 Week in Advance
 Prepare supplies and materials for presentation, place in
boxes Prepare supplies and materials for presentation, place in
boxes
Day of Session
 Arrive at least 30 minutes before scheduled start time Arrive at least 30 minutes before scheduled start time
 Check set-up and comfort of room Check set-up and comfort of room
 Place welcome and directional signs in the facility Place welcome and directional signs in the facility
 Make sure you know how to work video equipment Make sure you know how to work video equipment
1 Week after Session
 Send thank-you letter to on-site coordinator, participants Send thank-you letter to on-site coordinator, participants
A coalition is a group of organizations and individuals working
together for a common purpose. There are two types of coalitions or
partnerships:
Single-issue or event coalitions need to agree only on one
particular issue or event. The coalition dissolves when the issue is
resolved or the event takes place. Multi-issue coalitions focus on a set of related issues, such
as clinical trial access, health care for all, or quality of cancer
care. This more permanent type of coalition recognizes the value of
mobilizing together for action over a longer time. To be effective, a
multi-issue coalition should set a date for its work to be completed.
The coalition can always be reorganized if needed.
|
Why Form an Advocacy Coalition?
To build important links among community-based
organizations and clinical trial sites To share information with local organizations,
compare strategies, and unify positions on common issues To help:
Set priorities for action or funding Carry out an educational or outreach plan Broaden the development of new constituencies
|
The Value of a Coalition or Partnership
Coalition building is needed when one organization recognizes that
it alone does not have the capability or people-power to have a real
impact on an issue.
Clinical trial outreach is complex. Think of innovative ways to
reach underserved communities with clinical trial information. You
can be a catalyst for building important links among community-based
organizations and clinical trial sites.
Getting Started
Analyze Your Organization
Analyze your interests before asking other groups to join in your
efforts. Ask yourself:
What can be gained from joining with others? Will the advantages outweigh the disadvantages? How can we best communicate the demands of other groups
to our organization?
Forming an Advocacy Coalition--A Planning Guide
Step 1. Establish a Partnership Organizing Committee
The committee can be made up of leaders in your
organization. The committee manages the formation of the entire
partnership. Specific job responsibilities can be divided at the
first meeting.
Step 2. With the Group, Clarify Your Organization's Interest in
Developing a Community Partnership
Why do you want to advocate for access to clinical
trials? Why is it important? Which issues, questions, and behaviors around clinical
trials are of particular interest to you, and why? Do you need
more information on these topics? What don't you know about these issues, questions, and
behaviors around clinical trials? What questions do you need to
have answered? What outside resources can you tap to help you
understand the issues?
Identifying what you know about an issue also helps highlight what
you don't know-issues you can ask about when you get other
organizations involved.
Step 3. Identify Current Needs and Resources
When you start looking closely at clinical trial issues in your
community, you need detailed information about the needs of
individuals and the organizations that serve them, as well as the
resources that your community has available to solve those needs.
|
Needs can be defined as the gap between what a situation
is and what it should be. A need can be felt by an
individual, a group, or an entire community. It can be as
concrete as the need for more clinical trials or as abstract
as improved community understanding of clinical trials.
Examining needs can help you discover what is lacking, and
points you in the direction of future improvement.
|
Resources, or assets, can be used to improve quality of life. They
can be anything from people to places to organizations. Everyone is
an asset, and everyone has assets that can be used for community
building.
Identifying current needs and resources can help you:
Understand the environment where you'll be
working. Know how the community feels about an issue and what
members think needs to be done about it. Getting the opinions of
community members, identifying both the resources and limitations
of your area, will give you a holistic view of the issue. Make decisions about priorities for program or system
improvement. Once you assess the community, it is much easier to
make improvements that community members will notice and benefit
from.
This needs assessment process will be an ongoing part of forming
your coalition.
Step 4. Start with What You Know
After choosing an issue to focus on, you may find possible
solutions. Ask yourself:
What do we already know about the needs and
resources for clinical trials in our community? Has anyone else researched this topic in our community?
Can we rely on this other work to give us insight and answers? Are there experts in the community who can answer some
questions before we get started in forming the coalition? (Their
input may narrow the remaining questions you need to ask other
community members.)
Step 5. Identify the Key Organizations with Which You May Be
Working
Develop a list of organizations likely to want to participate in
the project. Be sure to include the institutions conducting clinical
research in your community. Possible participants may include those
listed on page 63.
Remember that this project presents an opportunity for you to
build important links among people with cancer, researchers, doctors,
and advocates.
Step 6. Determine What You Will Ask of Groups Who Join Your
Efforts
For example, each organization must be committed to:
Addressing clinical trial barriers in the
community Coordinating to solve the problem, not just to gain
public recognition The right of every other organization to be involved Open communication
Step 7. Write a Letter or E-mail Inviting Organizations to a
"Brainstorming" and Planning Session
The goal of this meeting is to figure out how community groups can
address local concerns associated with clinical trials. This
initiative may focus on collaboration among the groups. Keep in mind
that priorities may be different among the groups. This could be an
open meeting for discussion among interested parties. Publicize the
event through the media and at meetings.
Step 8. To Followup with All Participating Organizations,
Provide an Outline of Your Plans
Ongoing communication is critical to gaining community ownership
of this project.
Hold a Coalition Planning Meeting
Requirements for a Successful Meeting
Key requirements for a successful meeting include:
An accessible location and time A good facilitator, if possible from outside the
coalition, to help make sure all ideas are heard A good notetaker to record minutes, along with action
steps, and distribute them to attendees
The following are possible tasks for the first meeting.
Goal Setting
The meeting should have a goal. One example would be: "This
meeting will determine how community groups can collaborate on
addressing local concerns associated with clinical trials."
Team Building
Open the meeting with a team-building exercise. Pair people with
someone they don't know. Ask them to introduce themselves by telling
the other person about groups they belong to, as well as a skill or
talent they have. Ask people to introduce their partners by giving
their names, where they're from, groups they belong to, and a skill
or talent. Comment on the number of groups mentioned, the various
skills named, and how this is a beginning to building an effective
coalition/partnership.
Information Overview
Be prepared to:
Share clinical trials educational materials Discuss your goal in getting involved with advocacy Ask people to think about ways to spread the word
Brainstorming
In a brainstorming session, participants share their ideas or
suggestions for solving a problem. Discussion of each point occurs
after all ideas have been expressed. The atmosphere should be open
and encouraging.
Use a newsprint pad and marker and ask for a volunteer "recorder."
In the groups:
Discuss community perspectives on barriers to clinical trials.
How do they differ from the information presented? What other
concerns do members of the community have? Focus discussion on
barriers in the community, in institutions, and among potential
participants. Next, discuss possible solutions. Explain that this is a
participatory goal-setting process. Discuss potential projects to let
more people know about clinical trials.
|
Tip
You can use a buzz session in a coalition-building
meeting as well as for a community meeting. If you use a
buzz session instead of brainstorming, you may want to have
the groups use worksheets to discuss the issues. The
worksheets will help to determine community priorities for
this project.
|
Mission Statement
Meeting attendees should work to develop a mission statement for
the partnership. Examples of mission statements are:
To determine the issues of importance related to
clinical trials in our community, in order to develop a
community-based advocacy initiative To work together to reduce the barriers to clinical
trials inour community
The facilitator may need to further develop the statement and send
it to attendees for comment at the second meeting.
Finding Other Partners
Brainstorm to decide on other groups to invite to the next
meeting. List the following organization types and examples on
separate sheets of a newsprint pad:
When inviting groups to join the coalition, define the issue in a
way that appeals to their self-interest.
Followup Steps
Participants should resolve the following before the meeting
ends:
How to continue communication among coalition
members Who will facilitate future meetings (The facilitator
should invite other organizations to join the coalition's next
meeting. The group can elect someone to this position.) Who will coordinate an agenda for the next meeting
(Committee assignments are one way to develop different parts of
the agenda.) How additional input will be incorporated in the coalition's plans
|
To help you plan conducting community outreach and
education, see the
Plan for Action. 7
|
Ways to Work with the Media
What You Can Do
- Develop a media campaign
- Contact local media outlets about running a story related
to clinical trials
- Write a letter to the editor or an op-ed piece for your
local newspaper
Why?
To educate the public about clinical trials To encourage people to find out about trials To encourage local doctors to refer people to trials
Introduction
Media advocacy is the use of television, radio, newspapers,
magazines, or other media to help promote your mission. The media are
a primary source of health information. Using the media can help your
organization:
Change the way community members look at clinical
trials Create a reliable, consistent stream of publicity for your
organization's activities Motivate community members and policymakers to get involved
and learn more about clinical trials
Keep in mind that newspapers, television stations, and radio
stations have different audiences. Messages that work for one
audience may not work for another.
Education and Outreach in Action
|
A woman with colon cancer was very active in the
local PTA in her community. Many of the parents were
surprised when she told them about her decision to join a
clinical trial. One of the parents said, "I didn't know a
lot about clinical trials, but I saw how much the trial
is helping our friend. I figured that we had to let other
people know about it." The PTA approached the local
community newspaper to do a story on her experience in
the trial; which resulted in a five-part series.
|
Goals
Goals in using the mass media include:
Informing the public about issues related to clinical
trials in your community Recasting clinical trial barriers as concerns that affect
everyone, not just individuals Encouraging people to find out more about clinical trials
and to get involved
Timing
Good times to focus extra energy on the media include when:
Announcing a new project (for example, a new clinical trial
outreach plan) You have information that can be tied to a community
event The public or government officials are debating or
considering laws or activities that affect clinical trials
The Process
There are three different activities in media advocacy: agenda
setting, shaping the debate, and advancing a policy.
Agenda setting is what you accomplish when you influence:
What the media cover (media agenda) What people talk about (public agenda) How policymakers think about or act upon issues during
legislative sessions or in committee (policy or political agenda)
Agenda setting helps you make connections with the media and their
audiences. It also sets the stage for your next trick, shaping the
debate. To set an agenda:
Let the media and public know your concerns Get the public talking about what is important to
you Generate some sort of action (e.g., aim to influence
policy or get more people involved)
Shaping the debate is trying to change the way people talk about
clinical trials. The media often give tidbits of information about
clinical trial problems and then provide quick-fix solutions.
Traditionally, the media tell an audience:
"This is what the problem is"-providing
information about a problem "This is the solution"-usually summed up in a quick
health message such as, "Just say no."
Once you help to shape the way clinical trials are reported by the
media, then the community can work for better access to clinical
trials. Advancing a policy is a way to use the media to inform
policymakers about an issue. Media coverage created by advocates can
educate policymakers before they take action.
A media advocacy campaign is similar to a political campaign in
that you need to plan it carefully-identifying specific strategies
and tools to use-before taking any action. You need to understand how
you want the media to help you advance your goals.
Use the following steps to set up a media campaign:
Prepare your organization or your members. You will need to
designate a spokesperson to work with the media. Spokespeople need to
be prepared to speak with media representatives, using extra caution
with words and language that might be manipulated. Select your objectives. Ask yourself why you need to set up a
media campaign. Is a campaign the best way to meet your needs? What
are you going to use the media for? Do you want to inform the people
of important facts or do you want to get them involved? Do you want
to change policies or create new ones? Do you want to build support
for your goals and objectives? Identify your target audience. Anyone involved in health issues
can make a difference. Because people who live in one community can
have many different opinions and preferences, you can't reach
everyone with just one message. You will need to narrow your audience
and decide who you want to target:
Policymakers?
People who are facing a decision about treatment or
prevention options?
Groups who are undecided about supporting clinical
trials?
The general public, who doesn't know about clinical
trials?
Make a plan. Because media advocacy activities consume time and
money, it's important to put your best foot forward when you begin.
Carefully consider when to start your campaign, who you will contact
first, what issues you will tackle, and how you will present them.
What media would you like to use? Television, print media (such as
magazines, newspapers, and newsletters), radio, or billboards? Review your goals. Even though you put a lot of thought into
your media advocacy plan, be prepared to modify your goals. Remember,
the news can change quickly, and you need to be ready to react to an
opportunity, even one that doesn't fall under your organization's
typical scope of activities. Are your goals feasible? Keep your ear to the ground. Ask yourself what people talk
about, in your community and nationwide. What can polls and surveys
tell you about the hot topics? How much do people know about your
issues? What kinds of misconceptions or prejudices can you find in
public opinion?
|
Tips
Review local publications to understand better
what information each is likely to publish. Who is the
target audience? What types of articles are currently
available? Find out who reports on health and community
issues and go directly to him or her. Remember that reporters are always looking for
good news stories and you can serve as a resource to them.
Developing media opportunities is a challenging job, but
with the right preparation, you can soon become an
expert. Always be ready to arrange an interview with a
health care provider and a person with cancer. Factual
stories need a human-interest angle. Pitch stories in relation to health awareness
months. Ask producers of radio call-in shows to feature
clinical trials on one of their programs. Offer to provide
background information, sample questions and answers, and
people to interview. Use local organizations' media (e.g., newsletters,
Web sites, and listservs).
|
Press Releases
Press releases are one-page write-ups about your organization's
news. To be effective, they should be used sparingly. Press releases
are best used to announce an event, a coalition meeting, or other
strategy your group is employing to spread the word about clinical
trials.
Typically, press releases are faxed to the appropriate reporter or
editor.
The press release should be double-spaced and no more than a page
long.
Sample Press Release Format
|
Contact-
Be sure to include a
telephone number
|
|
 |
National Institutes of Health
EMBARGOED FOR RELEASE
1 p.m. EDT
Friday, November 01, 2002
NCI Press Office
(301) 496-6641
|
|
|
|
Headline-
When possible include
link to local event
|
|
|
National Cancer Institute Hosts Lecture, "Poor Whites and Health"
|
|
|
1st paragraph-
Interesting lead
sentence, general
description of event,
date, or place
|
|
|
J. Wayne Flynt, Ph.D., distinguished professor, Auburn University, Auburn, Ala., will
discuss the impoverishment of whites in America and the effect of poverty on
their medical care.
|
|
|
2nd and succeeding
paragraphs-
More description and
background. When
possible, obtain a
direct quote, local
interest angle.
|
|
|
Flynt received his Ph.D. from Florida State University in 1965,
specializing in Southern political history. His research interests
subsequently turned to poverty and religion. He has lectured at
universities across America and abroad.
Flynt is a community activist, serving on the American Cancer Society's Committee
for the Socioeconomically Disadvantaged. He is co-founder of the Alabama Poverty Project
and Sowing Seeds of Hope, an initiative to overcome poverty in Perry County, Ala.
Flynt is the author of 10 books, two of which have been nominated for Pulitzer Prizes.
In 2003-2004 he will serve as president of the Southern Historical Association,
the largest professional organization devoted to the study of Southern history
and culture.
This lecture is the second in a series presented by the National Cancer Institute's
Center to Reduce Cancer Health Disparities. This lecture series explores issues related to health
disparities and the unequal burden of disease in American society.
When: Tuesday, Nov. 5, 2002, 2 p.m.
Where: the lecture will take place in Masur Auditorium at the Warren G. Magnuson Clinical Center
on the National Institutes of Health (NIH) campus in Bethesda, MD.
|
|
|
For more information
provide a Web address
or telephone number
|
|
|
If you are interested in attending this lecture or require further
information, please contact (301) 496-6641.
|
|
Most media outlets are deluged with press releases. Consider
alternatives to the press release format:
Fact sheets-who, what, when, where, and why Position statements-a brief explanation of an issue and why
your group cares about it
Pros
Most nonprofit organizations rely on the news media to reach the
largest audience possible. Press releases:
Provide an excellent way to tip off journalists and editors
to your organization's news or upcoming activities Can be produced quickly in the event of breaking news Allow you to frame the issues Are easily distributed by mail, fax, or e-mail Encourage credible, objective journalists to report on your
activities
Cons
Press releases require skill to do well. Writing and distributing
a press release isn't enough. You have to work hard to form good
relationships with the media to make journalists more receptive to
your press releases and other efforts. And once you establish ties,
you have to work hard to maintain them. Remember that the attention
span of the news media is generally very short and that your story
has to compete with many others for attention.
Public Service Announcements
Public service announcements (PSAs) may be used to solicit
membership or to provide general information about your group and its
mission to spread the word about clinical trials. They are more
likely to be broadcast or printed if they announce a meeting or
public event.
|
Tip
Many media outlets run community calendar segments during
newsbreaks or talk programs. Call outlets and ask to insert
an item in their community calendar segments. They will
either take down the information over the phone or ask that
you provide it in writing.
|
When distributing your PSA, include a letter that sums up your
group's mission and why your PSA is important to your community. You
can also provide the public service director at the news outlet with
a press kit that includes more extensive information on your
group.
Radio
Radio PSAs should be submitted in a written script format or on a
professionally-produced tape. Written copies are often less expensive
and more effective, because they will be read by the radio announcer,
who is an experienced and familiar voice to the public ear.
Television
Keep in mind that television production can be very expensive and
requires experienced technicians and extensive camera equipment.
Don't forget about public access cable-more and more people are using
it. Using public access, you are most likely to get your message
broadcast without changes-a rare case where you have complete control
over your message.
Pros
PSAs:
Can give you free air time Can help long-range advocacy goals by helping to maintain
name recognition for your organization Are an easy way to spread information
Cons
You can't control the placement of your PSA, and competition for
"good airspace" can be fierce. In addition, PSAs:
Are labor intensive Offer less flexibility than paid advertisements (radio and
television stations may pick and choose which PSAs they wish to
use) Don't allow you to react quickly to breaking news because
they are hard to revise
Sample PSA Format
Usually it is best to simply provide the information for a PSA to
a media outlet, in a clearly readable format. In many cases a radio
or TV station will run a PSA exactly as you submit it. So, before you
fill in the blanks in the following sample, ask someone at the
station which format is best.
|
|
|
|
|
|
Sam Donaldson on Melanoma for NCI
|
|
|
Interesting lead
sentence
General description of
topic or event or who
is invited. You may
want to include a
sentence on history or
a local interest angle.
|
|
|
HI, I'M SAM DONALDSON. YOU MAY KNOW ME AS A NEWS ANCHOR, BUT I'M ALSO A MELANOMA
SURVIVOR. MELANOMA -- THE MOST SERIOUS TYPE OF SKIN CANCER -- CAN BE CURABLE
IF CAUGHT AND TREATED EARLY. SO YOU AND YOUR DOCTOR SHOULD EXAMINE YOUR SKIN
REGULARLY. OFTEN, THE FIRST SIGN OF MELANOMA IS A CHANGE IN SIZE, SHAPE, COLOR OR
FEEL OF AN EXISTING MOLE.
|
|
Final sentence-
Where to go for more
information.
|
|
|
FOR MORE INFORMATION, CALL THE NATIONAL CANCER INSTITUTE'S CANCER INFORMATION
SERVICE AT 1-800-4-CANCER OR VISIT NCI'S WEB SITE AT CANCER-DOT-GOV.
|
|
|
Tip
Remember to Keep It Short and Simple!
|
Letters to the Editor
The philosophy behind your local newspapers' letters-to-the-editor
pages can differ dramatically. The key variable is the size of the
paper's circulation.
Some editors of the letters page see it as a community bulletin
board where all sorts of opinions ought to be posted. This wide-open
policy typically is found at smaller papers, where they might be
struggling to fill the space they've allotted for letters. These
papers are a perfect opportunity for those interested in clinical
trial outreach and education.
In the midsize to large suburban and urban daily newspaper,
hundreds of letters may come in each day. For the editors of larger
papers, relevance is the key consideration. They want to print
feedback/criticism/praise for stories and opinion columns that have
appeared in their paper quite recently.
When you have evaluated the newspaper you are writing to and have
an idea of its circulation, you should begin to outline your topic.
First and foremost, know what you're writing about. Don't criticize a
newspaper's overall coverage of an issue unless you have truly read
every inch of coverage. Don't embarrass yourself and your
organization by claiming that a newspaper doesn't cover cancer
issues, for example, only to be presented with 30 clips of stories
printed over the past 2 years. If you're going to use the media, you
must first be a smart, consistent consumer of their products.
Keeping this in mind, it is best to focus your piece on a
particular story the paper has published:
Always quote the headline and date in your first or second
sentence. Stick to the facts and keep it dignified. Keep it brief, for the reader's sake. Four to six
paragraphs is the rule, with paragraphs consisting of only one or two
sentences each (generally 250-500 words max). If you can't make your point within these constraints,
consider writing a full-blown opinion column for the page, often
called the op-ed because it is published opposite the newspaper's
editorials. An op-ed column should consist of no more than 15
paragraphs, and 10 is a better length (500-800 words). Op-eds should
be signed by a prominent person in your community.
If possible, fax your letter or column to the editorial page. You
should identify a specific person to whom your letter should be sent.
The letter will get there more quickly, and 70 percent of editors say
they prefer faxes. You must sign your letter and include a daytime
telephone number.
If you have not seen your letter printed within a few days,
followup to inquire, politely. The answers you get will help you the
next time you write. Remember that no newspaper is obligated to print
your letter, but newspaper editors consider themselves obligated to
fairly and accurately present all sides of the issues. Use this ethic
to your advantage.
Sample Letter to the Editor
|
1st paragraph-
Begin by connecting to a timely event, previous
article, or statement. When possible, tie national events
into relevant stories or information about your
community.
|
|
|
Dear Editor:
Your most recent coverage of cancer in our community [April
11-Cancer Clinical Trials: False Promises] was an earnest attempt
to address the issues surrounding this topic. However, some important
facts about clinical trials were lost in the process. As [insert
what you are], I share the authors' concern about the potential
abuses in the clinical trials system and appreciate their commitment
to bring attention to this problem. But this year alone, it is
estimated that 553,400 people will die of cancer in the United
States-more than 1,500 people a day. By providing a one-sided and
sensationalistic view of a highly complex subject, you do a great
disservice to the important contributions of thousands of
hard-working and ethical researchers, as well as to clinical trial
participants.
|
|
|
2nd paragraph-
Make your point upfront.
|
|
|
The public's confidence in clinical research is seriously shaken
by stories like yours that report inadequacies in participant
protection without discussing the benefits of clinical trials.
Considering the thousands of people who take part in clinical trials
each year, breaches in protection are rare.
|
|
|
3rd paragraph-
Use facts to support your point. Make sure you get
your facts from a reliable source.
|
|
|
It is important to note that the number of studies executed
efficiently and ethically far outweighs these tragedies resulting
from clinical trials. We will never be able to answer the most
pressing questions about the causes of and treatments for cancer
without the scientific evidence produced through well-designed and
ethically executed clinical trials. Your readers should know that
many Federal regulations enforce participant protection rules. In
addition, in 2000, the U.S. Department of Health and Human Services
took additional steps to strengthen government oversight of medical
research and to reinforce clinical researchers' responsibility to
follow federal guidelines.
|
|
|
4th paragraph-
Be persuasive; appeal to readers' sense of justice,
decency, or empathy to strengthen your argument. Don't be
afraid to use a personal example as a way to support your
point of view.
|
|
|
Only 3 percent of adults with cancer currently participate in
clinical trials. How will we ever answer the most pressing questions
about the causes of and treatments for cancer without the scientific
evidence produced through well-designed and ethically executed
clinical trials?
|
|
|
5th and 6th paragraphs-
Say what readers should do, for your organization, for
your community, in support of clinical trials.
|
|
|
I firmly believe that people with cancer should have access to the
best treatments and be given the best possible chance for survival.
Clinical trials ultimately offer the best hope for many people, and
all must be offered the opportunity to participate. Participant
protection must be enforced in clinical research, and all
participants need to be appraised of the risks and benefits of
participating through existing informed consent laws.
We all need to know the facts about clinical trials. To learn more
about clinical trials, call NCI's Cancer Information Service at
1-800-4-CANCER or visit the NCI Web site, www.cancer.gov.
Sincerely,
|
|
 |
Ways to Work with Hospitals, Medical Centers, and Universities
What You Can Do
Help researchers design and conduct clinical trial
recruitment efforts Find ways to get primary care doctors, oncologists, and
oncology nurses to understand local clinical trial resources and to
refer people to trials Find out how you can serve on a local institutional review
board (IRB)
Why?
To help increase access and accrual to cancer clinical trials
Introduction
Many public institutions recognize the importance of including
nonscientists in the clinical trial review process and are including
patients and advocates as members of advisory committees and
planning/oversight groups that have a focus on clinical trials.
People from outside the research community play an important role
in program planning and implementation, and help to set
Education and Outreach in Action
|
A retired mail carrier helped her significant
other through cancer treatment. She read everything that
she got her hands on about chemotherapy and learned a lot
about cancer treatment.
Now that the cancer is in remission, she still logs
onto the National Cancer Institute's Web site for new
information. As a part of the local cancer support group,
she attended a local cancer conference. One of the
presenters discussed new medicine being studied in phase
3 trials, but mentioned in passing that the researchers
are having trouble getting patients to consider
participating in the trial.
During the "Q and A," she asked the presenter, "How
can patient groups help you get the word out about this
trial?" The group made a plan to meet with the
researchers to figure out how they could work
together.
Of the experience, the mail carrier says, "If I hadn't
made contact at that meeting, they would still be having
the same problems getting patients to consider
participating in this trial. Experts don't realize the
scope of patient organizations and all of the services we
have to assist them with their trials and any other
projects."
|
|
"I work in a breast cancer screening program,"
said a nurse from a Midwest health department. "Some
women are really worried about getting breast
cancer-they've lost a sister, a mother, an aunt to breast
cancer. Once they've gotten a normal mammogram report, I
tell them that they need to take care of themselves by
getting annual mammograms and clinical breast exams, and
I train them how to do breast self exams. But, I also
tell them that they may want to think about participating
in a breast cancer prevention trial." The health
department works with local physicians, the local medical
center, and some churches to let community members know
about opportunities to participate in breast cancer
prevention trials.
|
In addition to sponsoring community forums and information
sessions as outlined in other sections of this guide, many laypeople
have worked alongside researchers to advise them in their clinical
trial outreach and education efforts.
Points to keep in mind when assisting researchers with community
outreach include the following:
Community members who are known, trusted, and accountable
may be more effective and more believable to other community members
than a researcher. Community members can help advise researchers on ways to
present clinical trial information that complements the values people
in the community hold. Community members can help foster the development of
partnerships with organizations and the research institutions.
Tips to Find Out How You Can Help Researchers
Contact principal investigators or research nurses of local
institutions. To find out the kind of research being conducted in
your community:
Contact the research office of the institution. Use the NCI Research Portfolio to find out about research
going on in your community. The portfolio allows users to browse and
explore active NCI-supported research, including extramural and
intramural projects, by type of cancer or by type of cancer research.
Research projects (including treatment, prevention, and cancer
control studies) can also be sorted by research project title,
principal investigator, State, and institution. Visit
http://researchportfolio.cancer.gov.
Tips to Find Out How You Can Help Health Care
Professionals
Find ways to get primary care doctors, oncologists, and oncology
nurses to understand local clinical trial resources and to refer
people to trials:
Talk to your own primary care physician, oncologist and
nurse about resources available to them from the NCI, such as:
The PDQ clinical trials registry.
PDQ can be accessed by www.cancer.gov
and through 1-800-4-CANCER. The Cancer Information Service's Partnership Program. Through
this program, the Cancer Information Service reaches the medically
underserved, including minority groups and people with limited
access to health information and services, with cancer
information. The program can provide clinical trials training to
state and regional organizations working with these populations.
Call the Cancer Information Service at 1-800-4-CANCER or visit the
National Cancer Institute's Web site at www.cancer.gov/cis. The Cancer Trials Support Unit (CTSU). This is a pilot
project sponsored by the National Cancer Institute (NCI) that
supports a national network of physicians and patients to
participate in NCI-sponsored phase III cancer treatment trials.
For more information about joining, log on at www.ctsu.org
or call 1-888-823-5923. The NCI Research Portfolio 12. Find an oncologist who can present information on clinical
trials to primary care physicians and nurses in your community. Contact local chapters of the American Academy of Family
Physicians (www.aafp.org) and/or
the Oncology Nurses Society (www.ons.org)
to explore their knowledge of cancer clinical trials in your
community.
IRBs are made up of people who are qualified to evaluate new and
ongoing clinical trials on the basis of scientific, legal, and
ethical merit. The IRB determines whether the risks involved in a
study are reasonable with respect to the potential benefits. IRBs
also monitor the ongoing progress of the trial-from when it begins to
when it ends.
Federal regulations require that each IRB is made up of at least
five people; one member must be from outside the institution. IRBs
usually are made up of a mix of medical specialists and lay members
of the community. Many IRBs include members from diverse occupations
and backgrounds.
In most cases, IRBs are located where the study is to take place.
Most institutions that carry out clinical trials have their own
IRBs.
|
Typical Questions Addressed by an IRB
Are the risks to participants minimized as much as
possible through sound research design and the use of
safety-focused procedures? Are the risks reasonable in relation to the
anticipated benefits and the importance of the knowledge
that may result? Are participants selected fairly? Is a plan in place for seeking and documenting
participants' informed consent? Is the informed consent document both legally and
ethically sound? Have provisions been made for monitoring the data
collected to ensure the safety of participants as the trial
progresses? Have provisions been made to protect the privacy
of participants and the confidentiality of data collected
during the study?
|
To find out how you can serve on an IRB:
To learn about vacancies, contact administrators
(research or grant departments are good places to start) of local
hospitals, cancer centers, or universities. Some may have waiting
lists. Ask your doctor or nurse.
|
To help you plan for work with hospitals, medical centers, and universities see the Plan for Action. 5
|
Appendix
This worksheet is to help you determine how to accomplish your
goals for clinical trial outreach and education. We hope that this
planning will inspire you to take realistic steps towards your
goals. You may want to print this appendix for future reference.
Please be realistic in planning; you do not need to fill out every
page.
1. Remind yourself why you/your organization want(s) to get
involved in clinical trial outreach and education. Sometimes it can
be helpful to list these reasons before you plan your efforts.
List five reasons:
_____________________________________________________
_____________________________________________________
_____________________________________________________
_____________________________________________________
_____________________________________________________
2. Given your interest in the above topic areas, which of the
following activities do you feel ready to undertake during the next 6
months or next year?
p>_____________________________________________________
_____________________________________________________
_____________________________________________________
_____________________________________________________
_____________________________________________________
|
Read through the entire action plan before you begin to
check your interests. Be realistic about your other
commitments!
|
Ways to Educate Yourself
 Use NCI resources to keep up-to-date with new clinical trial
information, including www.cancer.gov Use NCI resources to keep up-to-date with new clinical trial
information, including www.cancer.gov
 Find local clinical trials in your community Find local clinical trials in your community
 Attend conferences, meetings, and workshops Attend conferences, meetings, and workshops
Ways to Educate Others, One-on-One
 Find opportunities to talk to others-neighbors, friends, and
coworkers-about clinical trials, and about the common myths
surrounding clinical trials, and where to find more information on
clinical trials Find opportunities to talk to others-neighbors, friends, and
coworkers-about clinical trials, and about the common myths
surrounding clinical trials, and where to find more information on
clinical trials
 Help others understand how to evaluate research in the news Help others understand how to evaluate research in the news
Ways to Conduct Community Outreach and Education
 Find opportunities and develop a strategy to speak about
clinical trials and/or: Find opportunities and develop a strategy to speak about
clinical trials and/or:
 Write articles for newsletters; post articles on Web sites Write articles for newsletters; post articles on Web sites
 Host an information session or community forum on clinical
trials Host an information session or community forum on clinical
trials
 Form a clinical trial advocacy coalition Form a clinical trial advocacy coalition
Ways to Work with the Media
 Develop a media campaign Develop a media campaign
 Contact local media outlets about running a story related to
clinical trials Contact local media outlets about running a story related to
clinical trials
 Write a letter to the editor or an op-ed piece for your local
newspaper Write a letter to the editor or an op-ed piece for your local
newspaper
Ways to Work with Hospitals, Medical Centers, and
Universities
 Help researchers design and conduct clinical trial recruitment
efforts Help researchers design and conduct clinical trial recruitment
efforts
 Find ways to get primary care doctors, oncologists, and oncology
nurses to understand local clinical trial resources and to refer
people to trials Find ways to get primary care doctors, oncologists, and oncology
nurses to understand local clinical trial resources and to refer
people to trials
 Find out how you can serve on a local institutional review board
(IRB) Find out how you can serve on a local institutional review board
(IRB)
3. What resources will you need to get these activities
accomplished? (Think about time, organizations, and individuals.)
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
4. Name five steps you will take to begin planning these
activities. Be as specific as possible.
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
5. What challenges do you see standing in your way of
accomplishing these activities?
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
6. How can you address these challenges? If you are working as a
part of a larger organizational effort, how can you get support from
your organization?
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
Education and Training. (2000). North Carolina State University
College of Agriculture and Life Sciences: North Carolina Cooperative
Extension Service. [Online], Available: www.ces.ncsu.edu/resources/education/
How You Can be an Advocate for Change: Action Against Cancer.
(1997). Washington, DC: Center for the Advancement of Health.
KU Work Group on Health Promotion and Community Development.
(2000). Community Tool Box. Lawrence, KS: University of Kansas.
[Online], Available: http://ctb.ukans.edu/tools/EN/section_1045.htm.
Stevens, G.L. (1990). A Process for Building Coalitions. Lincoln,
NE: Cooperative Extension, Institute of Agriculture and Natural
Resources, University of Nebraska. [Online], Available:
http://ianrwww.unl.edu/pubs/family/g988.htm
Susan G. Komen Breast Cancer Foundation. (1999). Educational
materials for affiliate distribution. Clinical Research Affiliates
Funding Trials (CRAFT).
|