|
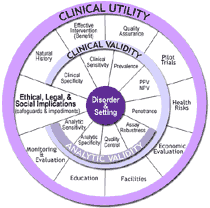 |
“Population-based Prenatal Screening for Cystic Fibrosis via Carrier Testing” is a draft document produced by the Foundation for Blood Research (Scarborough, Maine) under a cooperative agreement (UR3/CCU319352) with the CDC, National Office of Public Health Genomics. The ACCE project, named for the four components of evaluation—Analytic validity; Clinical validity; Clinical utility; and Ethical, legal, and social implications —is a model process for evaluating data on emerging genetic tests. The process consists of collecting, evaluating, and interpreting data about DNA-based tests and testing algorithms. |
“Population-based Prenatal Screening for Cystic Fibrosis via Carrier Testing” is the first systematic review using the model process, with additional reviews under development. The purpose of these reviews is to provide a comprehensive compilation of information and is intended for use by individuals and groups involved with shaping policy. The document is not, in and of itself, intended to recommend policy.
ACCE Project
Additional Information about the ACCE Project
Population-based Prenatal Screening for Cystic Fibrosis via Carrier Testing
ACCE Review: Population-based Prenatal Screening for Cystic Fibrosis via Carrier Testing
This document is comprised of seven, separate PDF files. You will need Acrobat Reader  to access these files. to access these files.
 |
Introduction  (386 KB) (386 KB)
A description of the model process and overview (Genetic Test Brief) |
 |
Disorder/Setting  (98 KB) (98 KB)
What is the test aimed at identifying and how will it be used? |
 |
Analytic validity  (649 KB) (649 KB)
How well does the test perform in the laboratory? |
 |
Clinical validity  (1740 KB) (1740 KB)
How well does the test perform in the clinical setting? |
 |
Clinical utility  (1031 KB) (1031 KB)
How useful is the test? |
 |
ELSI  (75 KB) (75 KB)
What are the ethical, legal and social implications associated with testing? |
 |
Glossary  (239 KB) (239 KB)
Terms used in the report |
|
|
Provides link to non-governmental sites and does not necessarily represent the views of the Centers for Disease Control and Prevention. |
|
|


