 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Prevention of Perinatal Group B Streptococcal Disease: A Public Health PerspectiveSummary Group B streptococcus is a leading cause of serious neonatal infection. Most neonatal GBS infections can be prevented through the use of intrapartum antimicrobial prophylaxis in women who are at increased risk for transmitting the infection to their newborns. However, despite clinical trials that demonstrate the effectiveness of intrapartum antibiotic prophylaxis, prevention strategies have not been implemented widely or consistently, and the incidence of neonatal GBS disease has not declined. To promote a coordinated approach to prevention among obstetric- and pediatric-care practitioners and among supporting clinical microbiology laboratory personnel, CDC has developed prevention guidelines in conjunction with experts from relevant disciplines and with representatives of the American College of Obstetricians and Gynecologists, the American Academy of Pediatrics, and other professional organizations. This report provides the epidemiologic basis for prevention protocols, summarizes results of clinical trials demonstrating the efficacy of intrapartum antimicrobial agents, examines limitations of different approaches to prevention, and presents guidelines for the prevention of GBS disease. CDC recommends use of one of two prevention strategies. In the first strategy, intrapartum antibiotic prophylaxis is offered to women identified as GBS carriers through prenatal screening cultures collected at 35-37 weeks' gestation and to women who develop premature onset of labor or rupture of membranes at less than 37 weeks' gestation. In the second strategy, intrapartum antibiotic prophylaxis is provided to women who develop one or more risk conditions at the time of labor or membrane rupture. Issues addressed by these prevention guidelines include the following: the appropriate clinical and laboratory methods required for prenatal screening programs designed to identify GBS carriers; risk conditions that indicate the need for intrapartum antibiotics; management of newborns whose mothers receive intrapartum antibiotic prophylaxis for GBS disease; and education of prenatal patients regarding GBS disease and the available prevention policy. These guidelines are intended for the following groups: a) providers of prenatal, obstetric, and pediatric care; b) supporting microbiology laboratories, hospital administrators, and managed-care organizations; c) childbirth educators; d) public health authorities; e) expectant parents; and f) advocacy groups for expectant parents. INTRODUCTION Since its emergence in the 1970s, group B streptococcal (GBS) disease has been the leading bacterial infection associated with illness and death among newborns in the United States. Newborns at increased risk for GBS disease are those born to women who are colonized with GBS in the genital or rectal areas. Colonized women who experience either a long duration of membrane rupture, premature delivery, or intrapartum fever are at particularly high risk for transmitting GBS infection to their infants during labor and delivery. Infants who have GBS disease can require prolonged hospitalization and expensive supportive therapy, and survivors may suffer permanent disability (e.g., hearing or visual loss or mental retardation). Many perinatal GBS infections can be prevented through intrapartum antimicrobial prophylaxis. GBS disease prevention programs require coordinated efforts among numerous specialties (e.g., providers of prenatal, obstetric, and pediatric care; supporting microbiology laboratories; managed-care organizations; quality assurance personnel; childbirth educators; and public health authorities). CDC developed these GBS disease prevention guidelines through critical analysis of clinical trial data and subsequent review of guidelines by consultants representing numerous disciplines. In December 1994, a draft version of the guidelines was published in the Federal Register. On March 10, 1995, CDC convened a meeting of clinical experts, public health authorities, representatives from professional organizations, and patient advocates to further consider these guidelines. The guidelines published in this report have been reviewed by both the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics. In addition, this document provides the epidemiologic basis for prevention protocols, summarizes results of clinical trials, examines limitations of various approaches to prevention, and presents prevention guidelines. BACKGROUND Group B streptococcus, or Streptococcus agalactiae, is a gram-positive coccus that causes invasive disease primarily in newborns, pregnant women, and adults with underlying medical conditions (e.g., diabetes mellitus). In infants, GBS disease is characterized as either early-onset (i.e., occurring in infants less than 7 days of age) or late-onset (i.e., occurring in infants greater than or equal to 7 days of age). Disease in infants usually occurs as bacteremia, pneumonia, or meningitis (1). Other syndromes (e.g., cellulitis and osteomyelitis) also can occur. Approximately 25% of the cases of neonatal GBS disease occurs in premature infants (2). In pregnant women, GBS infection causes urinary tract infection, amnionitis, endometritis, and wound infection; stillbirths and premature delivery also have been attributed to GBS (1). In nonpregnant adults, skin or soft tissue infection, bacteremia, genitourinary infection, and pneumonia are the most common manifestations of disease (2,3). The case-fatality rate for GBS disease is estimated to be 5%- 20% for newborns (1,2,4) and 15%-32% (2,3,5) for adults. A recent multistate, active surveillance system in a population of 10 million persons (2) demonstrated that 6% of early-onset GBS infections resulted in death. This case-fatality rate is lower than those reported previously (1,6), particularly the rates of 15%-50% observed in studies from the 1970s (7-9). This reduction in deaths most likely has resulted from improvements in neonatal care (10,11). EPIDEMIOLOGY Colonization The gastrointestinal tract is the most likely human reservoir of GBS, with the genitourinary tract the most common site of secondary spread (1). Colonization rates can differ among ethnic groups, geographic locales, and by age; however, rates are similar for pregnant and nonpregnant women (1,12-14). In most populations studied, from 10% to 30% of pregnant women were colonized with GBS in the vaginal or rectal area (12,13,15,16). Of all infants born to colonized parturients, approximately 1%-2% will develop early-onset invasive disease (1). The isolation rate of GBS from clinical specimens depends on several factors. Culturing specimens from both the anorectum and the vaginal introitus increases the likelihood of GBS isolation by 5%-27% over vaginal culture alone (15-17). The use of selective media (i.e., broths containing antimicrobial agents to inhibit competing organisms) is essential because they can increase the yield of screening cultures by as much as 50% (18,19). Appropriate selective broth media -- either SBM broth or Lim broth -- are commercially available. Incidence of Neonatal Disease Multistate, population-based methods of case-finding have been used to estimate the incidence of neonatal GBS disease in the United States. Age- and race-adjusted projections from multistate surveillance suggested that, in 1990, 7,600 episodes (i.e., incidence rate of 1.8 per 1,000 live births) and 310 deaths in the United States resulted from GBS disease among infants less than or equal to 90 days of age (2). Early-onset infections accounted for approximately 80% of neonatal GBS infections (2). Based on data from early case-series, long-term neurologic sequelae have been estimated to occur in 15%-30% of meningitis survivors (1); more recent estimates of the incidence and costs of sequelae from neonatal GBS disease are not available. Risk Factors for Perinatal GBS Disease Results of studies indicate that several obstetric, maternal, and neonatal factors increase the likelihood that early-onset GBS disease will occur in a newborn (Table_1). Infants born to women who were identified prenatally as GBS carriers had 29 times the risk of early-onset disease than did infants born to women whose prenatal cultures were negative (20). In the same study population, deliveries in which prematurity, longer duration of membrane rupture, or intrapartum fever occurred were approximately seven times more likely to be complicated by early-onset GBS disease (20). The incidence of GBS disease also is higher among infants born to mothers who are less than 20 years of age (21,22) or of black race (2,21). Other women with increased likelihood of delivering an infant who has invasive GBS disease are those with heavy colonization of GBS in genital cultures (8) or with low levels of anti-GBS capsular antibody (23) and women who previously delivered an infant who had GBS disease (24-26). Women with GBS bacteriuria during pregnancy usually are heavily colonized with GBS and appear to be at increased risk for perinatal transmission (27-30). Although multiple gestation has been suggested as a risk factor for GBS disease (31,32), only one study indicated increased risk independent of prematurity (31). Recent large studies failed to detect increased risk associated with multiple gestation (21,22). Risk factors identified for neonates include low birth weight and heavy surface colonization with GBS (8,33). Determinants of late-onset GBS disease are not well documented; however, some evidence suggests that late-onset disease can be acquired through either vertical or nosocomial transmission (6,34,35), although acquisition of disease from community sources also is possible (14). The role of GBS colonization in maternal infections was recently investigated (36). Factors that independently increased the risk for clinical amnionitis included GBS colonization, duration of membrane rupture (i.e., greater than 6 hours), duration of internal monitoring (i.e., greater than 12 hours), and number of vaginal examinations (i.e., more than six) (36). PREVENTION STRATEGIES Almost half the cases of invasive GBS disease occurs in newborns (2); therefore, efforts to prevent GBS disease have been concentrated on this group. Research has focused on either inducing protective immunity in the newborn (active and passive immunization) or eradicating colonization from the mother and/or newborn (chemoprophylaxis). Immunization Several studies have indicated that susceptibility to neonatal GBS disease is caused by a deficiency of maternal anticapsular antibody (23,37). Active maternal immunization may prevent peripartum maternal disease and neonatal disease by transplacental transfer of protective IgG antibodies (38). Several vaccines designed to induce antibodies against the polysaccharide capsule of GBS are being developed (39). These vaccines can potentially be used to prevent GBS disease in nonpregnant adults as well. The potential impact of effective vaccines may be limited because of reduced transplacental transport of protective antibody before 32-34 weeks' gestation and because of possible difficulty in making the vaccine available to pregnant women. Chemoprophylaxis Efficacy Studies Administering antimicrobial agents to pregnant women before the onset of labor or rupture of membranes is not likely to prevent neonatal GBS disease; however, some early studies have indicated that maternal colonization might be reduced by this method (40,41). In one study, asymptomatic pregnant women colonized with GBS were given oral antimicrobial drugs for 1 week during the third trimester; more than 30% of those treated were still colonized at delivery, and no substantial difference was observed in carriage of the organism at delivery between treated and untreated groups (42). Another study indicated that nearly 70% of colonized women who were treated for 12-14 days during the third trimester were colonized 3 weeks later and again at delivery even when their sex partners also had been treated (43). Postnatal chemoprophylaxis with intramuscular penicillin administered to infants just after birth also has been studied. Only one prospective, randomized, controlled study has been published in which blood cultures were collected from all newborns before chemoprophylaxis was conducted (44). In this study, in which only low-birth-weight infants were observed, no differences were observed between treated and untreated groups in either the incidence of early- or late-onset GBS disease or in mortality. Another study suggested that postnatal chemoprophylaxis with penicillin may decrease neonatal illness caused by GBS (45,46). However, no significant effect on overall mortality was observed, and mortality associated with penicillin-resistant pathogens was higher in the penicillin-treated group than in the control group (1.0 versus 0.4 deaths per 1,000 live births, p=0.06) (46). Because the majority of neonatal GBS infections are acquired in utero, antimicrobial agents administered to neonates, although useful for treatment, are unlikely to prevent the majority of GBS disease. Intrapartum chemoprophylaxis (i.e., administration of antimicrobial agents after onset of labor or membrane rupture but before delivery) is the most likely method of preventing both early-onset disease and maternal illness resulting from GBS; several antimicrobial regimens have been used for intrapartum chemoprophylaxis (Table_2). Several studies have indicated that intrapartum chemoprophylaxis decreases neonatal colonization (47- 51) and early-onset invasive disease (50-53) when administered to unselected pregnant women colonized with GBS (Table_3). Other studies have examined the use of intrapartum chemoprophylaxis for selected women colonized with GBS who were at increased risk for delivering an infant who had GBS disease. The only prospective, randomized, controlled clinical trial in which this approach was used focused on pregnant women colonized with GBS who experienced either preterm labor or membrane rupture (at less than 37 weeks' gestation) or prolonged rupture of membranes (greater than 12 hours before delivery) (54). In a preliminary study of the obstetric population in the same community, the incidence of early-onset GBS disease in this high-risk group was eightfold greater than among colonized women without any of these risk factors and 45-fold greater than among women with these risk factors who were not colonized (Table_1) (20,55,56). In the intrapartum chemoprophylaxis trial, colonized mothers with preterm labor or whose membranes ruptured greater than 12 hours before delivery were randomly selected to receive either intravenous ampicillin or no chemoprophylaxis. Infants born to mothers in the treatment (85 infants) and control groups (79 infants) differed substantially with respect to neonatal colonization (9% versus 51%, p less than 0.001) and early-onset invasive disease (0% versus 6%, p less than 0.02). Postpartum maternal febrile illness also was substantially reduced in the treatment group (p less than 0.04). Researchers estimated that this strategy could prevent at least 50% of the early-onset GBS infections in their patient population (54). Other studies also have documented the protective efficacy of intrapartum chemoprophylaxis administered to GBS carriers in certain high-risk groups (e.g., women with heavy genital colonization {57,58} and with rupture of membranes at less than or equal to 34 weeks' gestation and greater than 12 hours before labor onset {59}). The published studies of the efficacy of intrapartum chemoprophylaxis are summarized (Table_3). A recent meta-analysis of seven trials, which included studies of carriers with and without risk factors, estimated a 30-fold reduction in early-onset GBS disease with intrapartum chemoprophylaxis (60). Other investigators have argued that, because of the heterogeneity of therapeutic interventions and flaws in trial methods, combining results of GBS chemoprophylaxis trials is inappropriate (61). Identification of Carriers Most of the studies of intrapartum chemoprophylaxis have been used to evaluate its impact on subsets of women who had been identified as GBS carriers. Although the GBS carriage rate in pregnancy does not change with trimester (12,14), the duration of carriage is unpredictable (14), and prenatal screening cultures will not correctly identify all women with intrapartum GBS carriage. The later in pregnancy that cultures are performed, the closer the correlation with intrapartum culture results. However, scheduling routine cultures late in pregnancy means that some women who deliver prematurely will not be screened for GBS. In one study, when selective (antimicrobial-containing) broth medium was used and cultures were obtained from both the vagina and anorectum, only 7.4% of women with a negative culture at 26-28 weeks were found to carry GBS at delivery (16). The same study indicated that a single positive GBS culture during pregnancy was 67% predictive of a positive culture at delivery; the estimated sensitivity and specificity were 70.0% and 90.4%, respectively (16). Among 26 women whose prenatal cultures were obtained less than or equal to 5 weeks before delivery, there was 100% concordance with intrapartum culture status (i.e., no false-negative or false-positive prenatal cultures) (16). Data from follow-up of more than 5,000 deliveries by women who had had prenatal cultures for GBS indicated that 14 (88%) of the 16 infants who developed early-onset GBS disease were born to mothers who were detected prenatally as carriers (56). Optimal identification of GBS carriers is dependent on technique. The correlation of prenatal culture results with intrapartum GBS carriage is likely to be reduced substantially when screening does not incorporate appropriate culture sites (i.e., rectum and vaginal introitus), timing (as close to delivery as feasible), and culture medium (selective broth). Because cultures of specimens from the vagina and rectum are more sensitive than specimens from the cervix, (13), pelvic examination or visualization of the cervix by speculum examination should not be performed for collection of screening cultures. Appropriate broth media are commercially available (e.g., SBM broth or Lim broth). When a transport medium (e.g., Amies' medium) is used, group B streptococci from rectovaginal swabs will survive at room temperatures for up to 96 hours, permitting shipment from satellite clinics to a central microbiology laboratory. Selection Criteria Because of the findings of a randomized clinical trial, investigators recommended intrapartum chemoprophylaxis for those women identified through prenatal cultures as GBS carriers who subsequently develop one of the following signs: rupture of membranes greater than 12 hours before delivery, onset of labor or membrane rupture at less than 37 weeks' gestation, or intrapartum fever (temperature greater than 99.5 F {greater than 37.5 C}) (54). The American Academy of Pediatrics (AAP) supports the use of this strategy and added the following indications for intrapartum chemoprophylaxis: previous delivery of an infant with GBS disease and multiple-gestation pregnancy in a GBS carrier (62). In another report, investigators proposed an approach to GBS prevention that focused on prevention of disease associated with premature birth (63). That proposal suggested administering intrapartum antimicrobial agents to women with preterm labor or preterm rupture of membranes who were either colonized with GBS or whose colonization status was unknown. However, strategies designed to prevent infection only in preterm deliveries would have limited impact nationwide because less than 30% of all infants with GBS disease are preterm (2). A pragmatic approach to determining the need for antimicrobial prophylaxis was advocated by the American College of Obstetricians and Gynecologists (64,65). This strategy consists of using intrapartum antimicrobial agents for all women with one or more of the following conditions: preterm labor (at less than 37 weeks' gestation), premature rupture of membranes (at less than 37 weeks' gestation), prolonged rupture of membranes (i.e., greater than 18 hours before delivery), previous child affected by symptomatic GBS infection, or maternal fever during labor (65). This approach is less complex than protocols requiring either prenatal or intrapartum identification of GBS carriage; however, its efficacy against disease has not been evaluated in the controlled clinical trial setting, nor has the impact of the strategy on disease been measured in routine clinical practice. In Australia, researchers evaluated a strategy that recommended intrapartum penicillin prophylaxis for all women identified as GBS carriers through prenatal cultures collected at 32 weeks' gestation (53). Although the study was not a randomized trial, data from the study indicated a substantially lower incidence of early-onset GBS disease among the 30,197 women in the screened population compared with the 26,915 deliveries in the population that had no prenatal screening (p=0.04) (53). Clinical trials have not been conducted that directly compare the efficacy of the suggested prevention strategies. Conducting a study designed to find a statistically significant difference in efficacy may not be feasible; a recent article estimated that 100,000 women would be required for each study group of a randomized prospective trial comparing the efficacy of universal screening and selective intrapartum chemoprophylaxis with treatment based on risk factors alone (66). This limitation is reflected in a recent national consensus statement by the Society of Obstetricians and Gynaecologists of Canada and the Canadian Paediatric Society, which recommended use of either universal screening and selective intrapartum chemoprophylaxis or prophylaxis based on risk factors alone; the statement underscored the need for further prevention research (67). A combination of the features of a screening-based strategy and a strategy focused on prematurity offers a comprehensive approach to perinatal GBS prevention. This combination strategy relies on detection of GBS by rectovaginal cultures collected at 35-37 weeks' gestation; because some preterm deliveries will occur before culture results are available, the strategy also provides for intrapartum chemoprophylaxis for women who begin labor and/or membrane rupture before 37 completed weeks' gestation (i.e., unless results of the GBS culture are already available and are negative). Antibiotics would be offered intrapartum to all GBS carriers and to women at less than 37 weeks' gestation whose culture status is unknown. For women with rupture of membranes at less than 37 weeks who are not in labor, obstetric-care providers may choose either to begin antimicrobial prophylaxis until cultures are completed and negative or to delay beginning antimicrobial prophylaxis until a positive culture is identified. Data regarding which of these approaches is the most effective are not available. For women at greater than or equal to 37 weeks' gestation, if antenatal GBS culture status is unknown, antibiotics would be given if intrapartum fever is present or if membrane rupture has been greater than 18 hours. This precise strategy has not been directly evaluated in clinical trials, although treatment of all GBS carriers identified through screening at 32 weeks' gestation was evaluated in a trial in Australia (53). Data from another study demonstrated that the closer to delivery screening cultures are collected, the higher the predictive value of the test (16). In addition, this combination strategy and 18 other strategies have been evaluated by decision analysis (68); results indicate that the combination strategy would require intrapartum antimicrobial prophylaxis in 26.7% of deliveries and would prevent approximately 86% of the cases of early-onset disease. Comparative estimates for other strategies also are provided (Table_4). Adverse Effects Because a substantial proportion of pregnant women are colonized with GBS, administration of intrapartum chemoprophylaxis to all GBS carriers may cause an unacceptably high number of adverse reactions. Administering intrapartum antimicrobial agents to all women who are GBS carriers could result in approximately 10 deaths per year from anaphylaxis, assuming a GBS colonization rate of 25%, 4 million deliveries in the United States annually, and a rate of fatal anaphylaxis to penicillin of 0.001% (69). Another 0.7%-10% of women to whom prophylaxis is administered is expected to have less severe reactions (70). Severe complications can occur in the fetus even when maternal anaphylaxis is not life threatening (71). Widespread antimicrobial use also increases the risk for emergence of antimicrobial-resistant organisms. Infections with penicillin-tolerant GBS have been described (72-74), but GBS isolates have not developed clinically important resistance to penicillin. Development of antimicrobial resistance in other peripartum pathogens is a greater threat. In one study, investigators reported four episodes of adverse perinatal outcome caused by antimicrobial-resistant Enterobacteriaceae among women treated with ampicillin or amoxicillin for premature rupture of membranes (75). Restricting antimicrobial agents to selected populations at increased risk for delivering a newborn with GBS disease would decrease the likelihood of adverse reactions and antimicrobial-resistant infections. Administering intrapartum ampicillin to women identified prenatally as GBS carriers who have rupture of membranes for greater than 12 hours, labor or membrane rupture at less than 37 weeks' gestation, or intrapartum fever (temperature greater than 99.5 F {greater than 37.5 C}) would have required administering antimicrobial agents to 4.6% of the obstetric population served by one urban hospital (54). In another approach, administering antimicrobial agents to women with either labor or membrane rupture at less than 37 weeks' gestation who are intrapartum carriers of GBS or whose GBS status is unknown was estimated to require prophylaxis for 8.9% of parturients (63). Strategies that treat all GBS carriers (53) or all women with obstetric risk factors (e.g., prolonged membrane rupture and prematurity) (64) are estimated to require administering antimicrobial agents in a substantially higher proportion of deliveries. Penicillin G may be preferable to ampicillin for routine prophylaxis (76). Ampicillin and penicillin G have similar activity against GBS, and both cross the placenta and achieve bactericidal levels in fetal tissues. However, ampicillin has a broader spectrum of antimicrobial activity than penicillin; thus, widespread prophylaxis with ampicillin may be more likely to lead to selection of resistant organisms than would widespread use of penicillin (76). Implementation of Chemoprophylaxis Despite the encouraging results of efficacy studies, routine GBS screening and selective intrapartum chemoprophylaxis have not been widely adopted in the obstetric community (65,77). Practical problems include logistic concerns related to screening for GBS colonization and concern about the cost-effectiveness of implementing chemoprophylaxis. A strategy based on detecting colonization by prenatal screening and using these results to guide selective intrapartum chemoprophylaxis would not be effective for women who are not receiving prenatal care or whose prenatal records are not available to health-care providers at the time of delivery. Ideally, GBS carriage would be determined at the time of labor onset or at rupture of membranes. However, because identification of GBS by culture takes 24-48 hours, intrapartum culture results would not be available in time for intervention in most deliveries. Rapid detection of GBS antigen from vaginal specimens may identify GBS carriers when prenatal screening is not available (78). Although rapid tests for detection of GBS are specific and many recently developed tests can be performed in less than 1 hour, the sensitivity of rapid-detection tests has been variable and, often, unacceptably low (15%-74%) (78). Some rapid-detection kits are sensitive for detecting women who are heavily colonized. Three studies have confirmed the efficacy of intrapartum chemoprophylaxis administered to women identified by rapid-detection techniques as GBS carriers (57-59). However, because many infants with neonatal GBS disease are born to women who are lightly colonized (59,79), using currently available rapid-detection techniques to identify women for prophylaxis would probably prevent only a minority of GBS cases. The cost-effectiveness of selective intrapartum chemoprophylaxis for the prevention of GBS disease has been studied by using population-based rates of disease (80). The approach evaluated by two investigators (54) and later endorsed by AAP (62) was demonstrated to be cost-effective at the current rates of disease (80). The cost per case prevented (less than $35,000) was similar to that associated with maternal screening and intervention programs for other perinatal diseases (e.g., congenital syphilis) (81). Data from four other studies also have suggested that selective intrapartum chemoprophylaxis is cost-effective for the prevention of neonatal GBS disease (56,68,82,83). In particular, one study evaluated the combination strategy (i.e., prophylaxis for all women with preterm membrane rupture or labor whose culture status is unknown and for all GBS carriers identified through cultures obtained late in the pregnancy); this combination strategy was cost saving and was among the least expensive of 18 potential preventive strategies (68). Implementation of chemoprophylaxis is limited by two additional problems. First, clinicians have been concerned about adopting a strategy that inevitably will have failures (64). This concern may be influenced by the complexity of communicating risk information regarding GBS to women during pregnancy or by medicolegal considerations. Previous efforts to promote use of intrapartum chemoprophylaxis for only those GBS carriers who develop intrapartum complications (e.g., longer duration of membrane rupture, fever, or less than 37 weeks' gestation) appear to have been poorly accepted by both clinicians and patients. Some clinicians and patient groups have advocated informed consent for women who are GBS carriers. For example, women colonized with GBS would be informed of their risk for early-onset GBS disease (approximately 1 in 200). Also, the potential risks of intrapartum penicillin would be described (mild allergic reaction: approximately 1 in 10; anaphylaxis: approximately 1 in 10,000; and fatal anaphylaxis: approximately 1 in 100,000). Thus, patients could make an informed decision regarding intrapartum antibiotics. In instances in which this approach has been applied, few women choose not to receive antibiotics during labor. A second problem is that increasing intrapartum antimicrobial use may have a substantial impact on clinical management of the newborn (84,85). Some pediatricians routinely perform additional diagnostic tests on all infants whose mothers received intrapartum antimicrobial agents or observe these infants longer, leading to prolonged hospital stays for many newborns who are low risk for GBS disease (84). The decision of how to manage newborns whose mothers received intrapartum antimicrobial agents can be based on clinical manifestations, the infant's estimated gestational age, and the adequacy of intrapartum antibiotics (62). An algorithm for evaluating and managing infants after administration of maternal antibiotics has been developed as an empiric guide; however, clinical evaluation of alternative approaches remains appropriate at this time. Some of the challenges of instituting a prevention strategy are illustrated in two recent reports. In the first report, investigators initiated selective intrapartum chemoprophylaxis in their hospital in response to an increased rate of early-onset GBS disease (85). They enrolled 2,040 women, 332 (16.3%) of whom were colonized with GBS. Among women colonized at delivery, 122 (37%) had at least one obstetric risk factor and, according to the hospital's protocol, should have received intrapartum chemoprophylaxis. The circumstances of 16 (13%) women who did not receive intrapartum chemoprophylaxis illustrate some limitations of this strategy; these women did not receive intrapartum antimicrobial drugs because they either delivered less than 1 hour after arriving at the hospital (n=9), had had no prenatal care (n=3), or had had a negative prenatal culture but had a positive culture at delivery (n=4). Intrapartum chemoprophylaxis was not administered to an additional 17 (14%) women because protocol was not followed. Eleven infants had early-onset GBS disease; two had received one dose of intrapartum chemoprophylaxis and were asymptomatic; and nine were born to carriers with risk factors who did not receive intrapartum chemoprophylaxis. No affected infants were born to colonized women without risk factors or to women whose prenatal screening cultures were negative for GBS. One woman who received intrapartum chemoprophylaxis developed a rash and transient hypotension and was delivered by cesarean section because of transient fetal bradycardia. The study suggested that selective intrapartum chemoprophylaxis was effective in preventing early-onset GBS disease, that the infants of colonized women without labor complications are at low risk for disease, and that administering intrapartum antimicrobial agents is not without risks. The second report illustrates that a prevention strategy employing selective intrapartum chemoprophylaxis is not easily implemented (86). In this study, which was conducted in an academic setting, 114 (80.3%) of 142 women who had positive GBS screening cultures and who developed risk factors at delivery received intrapartum antimicrobial drugs. Reasons for failing to receive appropriate treatment included failure to follow protocol, marginal indications for chemoprophylaxis, or patient refusal. Early results of the study suggest that institution of the protocol was associated with a downward trend in the rate of disease. DISCUSSION Group B streptococcal disease continues to be a major cause of illness and death among newborns despite clinical advances in the last two decades. Major risk factors for early-onset neonatal GBS disease include maternal GBS colonization, longer duration of membrane rupture, intrapartum fever, less than 37 weeks' gestation, GBS bacteriuria during pregnancy, and previous delivery of an infant who had GBS disease (6,21,25,27). A substantial number of cases of early-onset neonatal GBS disease can be prevented by administering prophylactic antimicrobial agents during labor (56). Furthermore, obtaining specimens from the lower vagina and rectum during the third trimester for culture in selective broth media will identify the vast majority of women who are colonized with GBS at delivery. Increasing evidence suggests that treating GBS-infected newborns is more costly than preventing the infection and that well-implemented prevention programs can substantially reduce illness and death resulting from GBS disease (56,68,80,82,83). Any prevention program for GBS must be implemented carefully. For example, failure to use optimal culture methods can compromise the effectiveness of screening strategies. A recent survey of obstetric-care providers in Georgia identified several barriers to effective prevention activities. For example, only 9% of providers who obtained screening cultures followed recommended procedures and 32% administered antimicrobial agents prenatally when carriage was detected even though 93% of providers stated they knew such treatment to be ineffective (87). An optimal prevention strategy currently is a combination of routine prenatal screening for GBS colonization late in pregnancy and empiric management of those preterm deliveries that occur before the GBS culture is available. This approach includes collecting rectovaginal cultures at 35-37 weeks' gestation and offering intrapartum treatment to all carriers. Because some preterm deliveries occur before culture results are available, the strategy also provides for intrapartum chemoprophylaxis for women who begin labor and/or membrane rupture before 37 completed weeks' gestation (unless results of the GBS culture are already available and are negative). Criticism of previous screening-based strategies has included concerns regarding the predictive value of prenatal cultures, the ethical and legal difficulties inherent in withholding intrapartum antibiotics from GBS carriers without risk factors, and the pressure experienced by clinicians to respond to antenatal GBS carriage results by treating with oral antimicrobial agents remote from delivery. Critics of empiric approaches have been concerned that despite use of intrapartum antimicrobial prophylaxis in a substantial proportion of deliveries, the strategy does not prevent the 25% of all early-onset GBS cases that occur in GBS carriers without risk factors. Further, empiric approaches do not address the strong preference of some patients and providers for prenatal screening cultures. Rationale for Screening-Based Approach to Prevention The combination of late prenatal-screening cultures and empiric management offers several potential advantages over exclusive reliance on either a screening-based approach or an empiric approach. Because the combination strategy schedules collection of a GBS culture at 35-37 weeks' gestation, the concordance of prenatal and intrapartum GBS carriage status will be high. Thus, false-negative prenatal culture results will be minimized (i.e., few women with negative cultures at 35-37 weeks' gestation will become colonized with GBS by the time of delivery), and nearly all women identified antenatally as carriers will still be carrying the organism at delivery, potentially justifying exposing this group to adverse effects associated with antibiotics. Even women who begin prenatal care late in gestation should be eligible for late screening. By avoiding collection of cultures earlier in gestation, this strategy should reduce pressure on clinicians to treat GBS carriage antenatally. Because all GBS carriers will be offered intrapartum chemoprophylaxis, clinicians will not need to wait for the development of intrapartum risk factors in a GBS carrier (e.g., duration of membrane rupture greater than 18 hours or intrapartum fever). Intrapartum prophylaxis for most women can therefore begin earlier, so that most GBS carriers will be administered antimicrobial agents greater than 4 hours before delivery or in time for adequate antibiotic levels to be reached in amniotic fluid. In one analysis, the combination of late prenatal screening and intrapartum prophylaxis of carriers and preterm deliveries was estimated to prevent approximately 86% of early-onset disease, was cost-saving compared with no intervention, and was among the least expensive of 18 potential preventive strategies (68). Large-scale clinical experience with the combination approach previously described is not yet available, although the strategy has been applied in several institutions. The theoretical advantages of this approach address most concerns that have been raised regarding the prevention strategies previously considered. Although antimicrobial prophylaxis will be used in a high proportion of deliveries, some clinicians and patients have found selective approaches (e.g., treating only carriers with risk factors) unacceptable, because treatment is withheld from a group of women with moderately increased risk of GBS disease -- GBS carriers without intrapartum risk factors. Assessment of the current situation suggests that any prevention program that is acceptable to both clinicians and their patients will involve using antimicrobial prophylaxis in a substantial proportion of deliveries; thus, the strategy that directs use of these antimicrobial agents to women at the highest risk for GBS disease seems reasonable. Communication mechanisms and information systems should be developed and monitored to ensure that prenatal culture results are available at the time and place of delivery. Research into Enhancing Prevention A program combining late prenatal screening for GBS with empiric treatment of women with preterm deliveries is not a permanent solution to the problem of neonatal GBS disease, although it may be a reasonable option that is available now. A more sensitive rapid-screening test for GBS that could accurately detect women who carry GBS at the time of delivery would obviate the need for prenatal screening. Sensitive intrapartum testing also would permit detection of GBS carriage among women who did not receive adequate prenatal care. Because an intrapartum test might detect a higher proportion of women who carry the organism at delivery and will not detect women who only carry the organism earlier in pregnancy, intrapartum use of a sensitive rapid-detection test could make a prevention program simpler and more efficient. An adequate rapid-detection test must be a) sensitive (e.g., 85%-90% compared with culture in selective broth media); b) rapid (results available to clinicians in time for antibiotics to be given before delivery); and c) convenient (for integration into routine laboratory use). Even a highly sensitive rapid-detection test would not be adequate if results were not available to clinicians 24 hours a day, 7 days a week. Development of a vaccine against GBS that is highly immunogenic in women and permits transplacental transfer of protection to the fetus also would eliminate the need for prenatal screening and could potentially address the problem of late-onset GBS disease, which intrapartum antimicrobial agents do not prevent. Surveillance and Evaluation Because incidence may vary widely, state or local health departments or groups of affiliated hospitals should consider either establishing surveillance systems for neonatal GBS disease or reviewing data from existing systems to identify the current magnitude of disease and gain further information for evaluating the effectiveness of prevention measures. In hospital settings, prevention programs should monitor the occurrence of adverse reactions to chemoprophylaxis, the emergence of perinatal infections caused by antimicrobial-resistant organisms, and the impact of obstetric antimicrobial use on pediatric management protocols. RECOMMENDATIONS Enhanced communication among personnel in multiple disciplines is needed to ensure that programs for prevention of GBS disease succeed. Open communication between clinicians and patients is a critical component of GBS disease prevention. An informational brochure for pregnant women on GBS is available through CDC (Childhood and Respiratory Diseases Branch, Division of Bacterial and Mycotic Diseases, National Center for Infectious Diseases, Mailstop C09, Atlanta, GA 30333; Internet address: http://www.cdc.gov/ncidod/diseases/bacter/strep_b.htm. The following recommendations for the prevention of GBS disease will need periodic reappraisal to incorporate advances in technology or other refinements in prevention strategies.
Screening-Based Approach. All pregnant women should be screened at 35-37 weeks' gestation for anogenital GBS colonization (Figure_1). Patients should be informed of screening results and of potential benefits and risks of intrapartum antimicrobial prophylaxis for GBS carriers. Information systems should be developed and monitored to ensure that prenatal culture results are available at the time and place of delivery. Intrapartum chemoprophylaxis should be offered to all pregnant women identified as GBS carriers by culture at 35-37 weeks' gestation.
For intrapartum chemoprophylaxis, intravenous penicillin G (5 mU initially and then 2.5 mU every 4 hours) should be administered until delivery (Box 2 Table_B2). Intravenous ampicillin (2 g initially and then 1 g every 4 hours until delivery) is an acceptable alternative to penicillin G, but penicillin G is preferred because it has a narrow spectrum and thus is less likely to select for antibiotic resistant organisms. Clindamycin or erythromycin may be used for women allergic to penicillin, although the efficacy of these drugs for GBS disease prevention has not been measured in controlled trials. (Note: Penicillin G does not need to be administered to women who have clinical diagnoses of amnionitis and who are receiving other treatment regimens that include agents active against streptococci {e.g., ampicillin or clindamycin}.) 5. Routine use of prophylactic antimicrobial agents for infants born to mothers who received intrapartum prophylaxis is not recommended. However, therapeutic use of these agents is appropriate for those infants suspected clinically of having sepsis. Additional research is needed to determine algorithms for management of infants born to mothers who receive intrapartum antimicrobial prophylaxis. One algorithm for empiric management of these newborns is provided (Figure_3). Other management approaches, developed by individual physicians or institutions, may be appropriate alternatives. 6. Local and state public health agencies, in conjunction with appropriate groups of hospitals, should consider establishing surveillance to monitor the incidence of neonatal GBS disease, occurrence of adverse reactions to antimicrobial prophylaxis, and the emergence of perinatal infections caused by penicillin-resistant organisms. Investigations designed to evaluate and compare these two strategies and others are needed. Such studies will require the participation of multiple institutions and should evaluate multiple outcomes (e.g., perinatal GBS infections, adverse reactions to antimicrobial prophylaxis, and perinatal infections caused by penicillin-resistant organisms). Characterization of protocol failures may contribute to improvement of future prevention strategies. References
+------------------------------------------------------------------- -------+ | | | Erratum: Vol. 45, No. RR-7 | | | | SOURCE: MMWR 45(31);679 DATE: Aug 09, 1996 | | | | The MMWR Recommendations and Reports, "Prevention of Perinatal | | Group B Streptococcal Disease: A Public Health Perspective," | | contained two errors. | | | | Page 17, Box 1: Item 3 should read: | | 3. Remove the swabs from the transport medium and inoculate both | | swabs together into selective broth medium. Todd-Hewitt broth | | supplemented with either colistin (10 ug/mL) and nalidixic acid (15 | | ug/mL) or with gentamicin (8 ug/mL) and nalidixic acid (15 ug/mL) | | may be used; appropriate commercially available options include Lim | | or SBM broth. | | | | Page 20: Figure_3 contained an arrow pointing in the incorrect | | direction. The corrected Figure_3E appears below. | | | +------------------------------------------------------------------- -------+ Table_1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 1. Risk for early-onset group B streptococcal (GBS) disease (20), by prenatal maternal
colonization status and intrapartum risk factors
===============================================================================================
No. of
episodes Early-onset
early-onset No. of Attack Rate Deliveries GBS cases
Risk status * GBS disease deliveries (per 1000 births) (%) (%)
-----------------------------------------------------------------------------------------------
Total population 16 5,292 3.0 100.0 100.0
Colonization+ 14 1,029 13.6 19.4 87.5
Colonization- 2 4,263 0.5 80.7 12.5
Risk factors+ 11 1,311 8.4 24.7 68.8
Risk factors- 5 3,981 1.3 75.2 31.3
Colonization+
Risk factors+ 10 245 40.8 4.6 62.5
Colonization+
Risk factors- 4 784 5.1 14.8 25.0
Colonization-
Risk factors+ 1 1,066 0.9 20.0 6.2
Colonization-
Risk factors- 1 3,197 0.3 60.0 6.2
-----------------------------------------------------------------------------------------------
* Colonization assessed by prenatal rectovaginal cultures. Risk factors defined as rupture of
membranes longer than 12 hours, <37 weeks' gestation, or intrapartum temperature>99.5 F
(>37.5 C). "+" refers to present; "-" refers to absent.
===============================================================================================
Return to top. Table_2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 2. Summary of antimicrobial regimens used for studies of intrapartum chemoprophylaxis for
group B streptococcal (GBS) disease
===================================================================================================
Agent Dose and schedule * Comments +
---------------------------------------------------------------------------------------------------
Ampicillin (47) 500 mg IV every 6 hrs 30/34 received only one dose before delivery
Ampicillin (52) 500 mg IV Administered every 6 hrs
Benzyl penicillin (48) 600 mg IM every 8 hrs Erythromycin 100 mg IM for women
allergic to penicillin
Ampicillin (50) 500 mg IV every 6 hrs 46/57 received only one dose before
delivery
Penicillin (53) 1 mU IV every 6 hrs
Ampicillin (54) 2 g IV load then 1 g IV Mean duration of prophylaxis 5.4 hrs
4 hrs
Penicillin G (58) 5 mU IV every 6 hrs If labor lasted 18 hrs, then penicillin
V 1 mU should be administered orally
every 8 hrs after initial IV therapy
Ampicillin (59) 1 g IV every 6 hrs
Ampicillin (57) 1 g IV every 6 hrs Ampicillin levels measured in eight
mother-infant pairs
---------------------------------------------------------------------------------------------------
* IV = intravenous; IM = intramuscular.
+ mU = million units.
===================================================================================================
Return to top. Table_3 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 3. Summary of trials employing intrapartum chemoprophylaxis for prevention of
neonatal colonization and early-onset group B streptococcal disease
====================================================================================================
Neonatal colonization Early-onset disease
Study design, Case selection ----------------------- -----------------------
control selection * criteria + IC & No IC P value IC No IC P value
----------------------------------------------------------------------------------------
R (47) I 0/34 14/24 0.001 0/34 0/24 NA @
Random
P/R (52) I 4/57 62/136 <0.01 0/57 9/136 0.06
Nonrandom
P (48) PC 0/38 17/49 0.001 ND ND NA
Random
P (50) I or PC 2/60 24/65 <0.01 0/60 3/65 0.14
Random
P (53) PC ND ND NA 16/ 27/ 0.04
Nonrandom 30,197 26,915
P (54) PC and
Random Pre/PROM 8/85 40/79 0.001 0/85 5/79 0.02
P (54) PC and
Nonrandom Pre/PROM 5/82 102/233 <0.01 0/82 7/233 0.02
P (58) Heavy PC ND ND NA 1/88 10/111 0.03
Random
P/R (59) Light I and
Nonrandom PPROM ND ND NA 0/29 6/37 0.03
P/R (59) Heavy I and
Nonrandom PPROM ND ND NA 0/7 7/11 0.01
P (57) Light PC 0/98 35/98 <0.01 0/98 0/98 NA
Random
Heavy PC 0/37 24/30 <0.01 0/37 3/30 0.09
----------------------------------------------------------------------------------------
* R=retrospective; P/R=prospective case selection, retrospective control selection; P=prospective.
+ I=intrapartum colonization; PC=prenatal colonization; Pre/PROM=preterm labor (<37 weeks'
gestation) or prolonged rupture of membranes (>12 hours); PPROM="preterm premature rup-
ture of membranes" (i.e., rupture of membranes at <=34 weeks' gestation and >12 hours before
onset of labor).
& IC=Intrapartum chemoprophylaxis; ND=not done.
@ NA=not applicable.
====================================================================================================
Return to top. Table_4 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
TABLE 4. Estimated impact of several strategies for the use of intrapartum antimicrobial
prophylaxis (IAP) against early-onset group B streptococcal disease in a hypothetical
population (adapted from 68).
===========================================================================================
Proportion of early-onset Proportion of deliveries
GBS disease prevented receiving IAP
Prevention strategy (%) (%)
-------------------------------------------------------------------------------------------
Prenatal culture at 35-37 weeks' 86.0 26.7
gestation; IAP for preterm
deliveries and all GBS carriers *
Prenatal culture at 26-28 weeks' 50.7 3.4 +
gestation; IAP for GBS carriers
who develop intrapartum risk
factors (e.g., fever, prolonged
rupture of membranes, <37 weeks'
gestation) (54)
No prenatal cultures; IAP for all 68.8 18.3 &
women with intrapartum risk
factors (e.g., fever, prolonged
rupture of membranes, <37 weeks'
gestation) @
-------------------------------------------------------------------------------------------
* Combination strategy; refer to Figure 1.
+ Percentage was estimated for a hypothetical population (68); actual proportion of
deliveries among who had prenatal screening cultures positive for GBS and who also
developed intrapartum risk factors was 4.6% (20).
& Percentage was estimated for a hypothetical population (68); actual proportion of
deliveries among women who had intrapartum risk factors was 24.7% (20).
@ Empiric strategy suggested by the American College of Obstetricians and Gynecologists
in 1993 (64, 65); refer to Figure 2.
===========================================================================================
Return to top. Table_B1 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size. BOX 1. Procedure for collecting and processing clinical specimens for culture of group b Streeptococcus ============================================================================= 1. Obtain one or two swabs of the vaginal introitus and anorectum. Cervical cultures are not acceptable; a speculum should not be used for culture collection. 2. Place the swabs into a transport medium. The swabs in a transport medium will maintain GBS viability for up to 4 days at room temperature or under refrigeration. Appropriate nonnutritive moist swab transport systems (e.g. Amies') are commercially available. 3. Remove the swabs from the transport medium and inoculate both swabs together into selective broth medium. Todd-Hewitt broth supplemented with either colistin (10 g/mL) and nalidixic acid (15 g/mL) or with gentamicin (8 g/mL) and nalidixic acid (15 g/mL) may be used; appropriate commercially available options include Lim or SBM broth. 4. Incubate selective broth for 18-24 hrs. Subculture the broth to sheep blood agar plate. 5. Inspect and identify organisms suggestive of GBS (beta hemolytic or nonhemolytic, gram-positive and catalase negative). If GBS is not identified after incubation for 18-24 hrs on sheep blood agar plate, reincubate and inspect at 48 hrs to identify suspected organisms. 6. Various slide agglutination tests or other tests for GBS antigen detection (e.g., genetic probe or fluorescent antibody) may be used for specific identification, or the CAMP test may be employed for presumptive identification. ============================================================================= Return to top. Figure_1 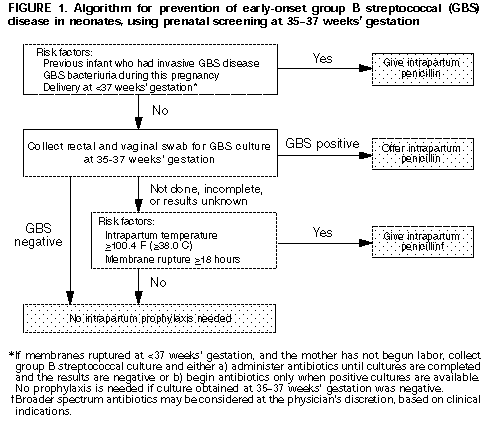 Return to top. Figure_2 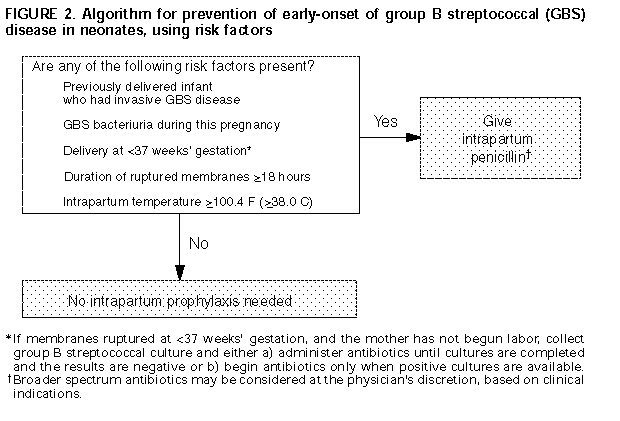 Return to top. Table_B2 Note: To print large tables and graphs users may have to change their printer settings to landscape and use a small font size.
BOX 2. Recommended regimens for intrapartum antimicrobial prophylaxis for
perinatal group B streptococcal disease
=============================================================================
Recommended Penicillin G, 5 mU IV
load, then 2.5 mUs IV every
4 hrs until delivery
Alternative Ampicillin, 2 g IV load, then 1 g
IV every 4 hrs until delivery
If penicillin-allergic
Recommended Clindamycin, 900 mg IV every 8 hrs
until delivery
Alternative Erythromycin, 500 mg IV every 6 hrs until delivery
-----------------------------------------------------------------------------
* Note: If patient is receiving treatment for amnionitis with an
antimicrobial agent active against group B streptococci (e.g., ampicillin,
penicillin, clindamycin, or erythromycin), additional prophylactic
antibiotics are not needed.
=============================================================================
Return to top. Figure_3 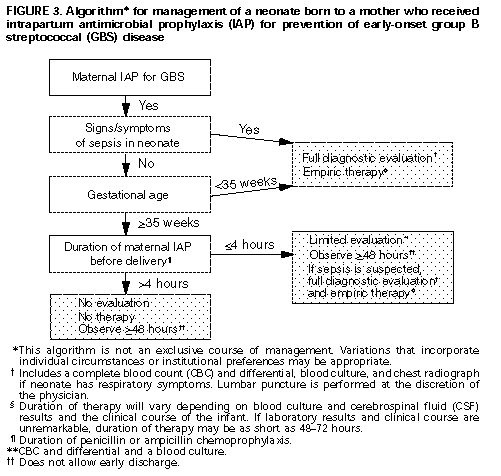 Return to top. Figure_3E 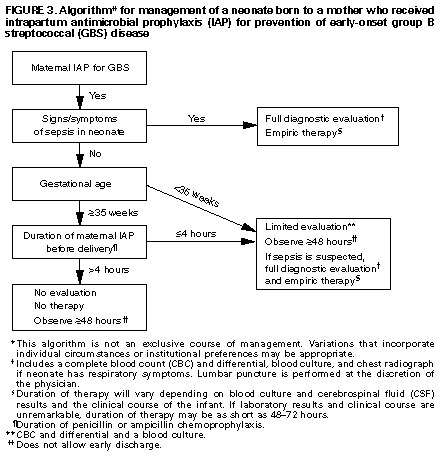 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|