● Drug-Resistant
Tuberculosis: A Fact Sheet for Botswana

● TB Publications
TB Control in Botswana
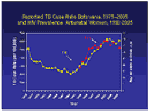
 TB is a major health problem in Botswana. The Ministry of Health established the Botswana National Tuberculosis Control Program (BNTP) in 1975, which initiated a decline in TB cases. However, since 1989, Botswana has experienced a three-fold increase in TB incidence and now has one of the highest rates of TB in the world. The increase has largely been attributed to the country's increasing HIV epidemic. It is estimated that 60-86% of TB patients in Botswana are currently HIV co-infected. TB is a major health problem in Botswana. The Ministry of Health established the Botswana National Tuberculosis Control Program (BNTP) in 1975, which initiated a decline in TB cases. However, since 1989, Botswana has experienced a three-fold increase in TB incidence and now has one of the highest rates of TB in the world. The increase has largely been attributed to the country's increasing HIV epidemic. It is estimated that 60-86% of TB patients in Botswana are currently HIV co-infected.
BOTUSA in Botswana
At the invitation of the Ministry of Health of Botswana, BOTUSA was
established in 1995 as a TB/HIV epidemiologic research partnership. The TB
efforts at BOTUSA are a collaborative effort of the BNTP, the National TB
Reference Laboratory and the Division of TB Elimination at the U.S. Centers
for Disease Control and Prevention (CDC). The goal of this partnership is to
generate information that will improve TB control efforts in Botswana and
elsewhere in the face of the TB-HIV co-epidemic. Past Achievements of
the BOTUSA Partnership
- Study on causes of treatment delays among TB patients and risk
factors for TB transmission;
- Development of an electronic TB register for TB surveillance;
- Survey of physician practices for TB diagnosis;
- Postmortem studies in adults and children;
- Cost-effectiveness of home-based care; and
- Assessments of TB prevalence in refugee and prison populations.
More Recent BOTUSA Studies (2003-2007)
- Assessment of the efficacy of a new agent (CB-18) to enhance sputum
smear testing among adult TB patients;
- Annual risk of TB infection in Botswana was conducted through a skin
test survey of 10,000 school-aged children;
- Study of risk factors for malabsorption of anti-TB drugs in
HIV-infected TB patients;
- In 1995-1996, 1999, and 2002 CDC conducted national anti-TB drug
resistance surveys in collaboration with the BNTP. A survey was launched
in 2008 to identify MDR and XDR-TB.
The IPT Trial (2004-present)

With a staff of 40, the TB/HIV Research Team at BOTUSA is engaged in a
2000-person clinical trial that is aimed at determining the efficacy of
continuous isoniazid TB preventive therapy among people living with HIV/AIDS
(PLWHA) as compared with the standard 6-month regimen. This double-blind
randomized placebo controlled study is being conducted in two cities. The
trial has received high marks from the independent Data Safety Monitoring
Committee as well as the Endpoint Committee. Nested within this study is an
investigation of the accuracy of a new interferon gamma assay (Quantiferon-Gold)
for the diagnosis of latent TB infection among PLWHA. Technical Support
for Botswana's National TB Program
 BOTUSA's TB/HIV
Research section provides technical support to the CDC's
Global AIDS Program in Botswana, the Ministry of Health's National
Tuberculosis Programme and the National Tuberculosis Reference Laboratory.
These activities include membership in the National TB-HIV Advisory
Committee, membership in the MDR TB Advisory Committee, regular meetings
with the National Tuberculosis Programme, a National TB Programme Review in
2006, revision of the 2007 National TB Programme manual, and support to
develop and conduct an evaluation of the national isoniazid preventive
therapy program in 2008. BOTUSA's TB/HIV
Research section provides technical support to the CDC's
Global AIDS Program in Botswana, the Ministry of Health's National
Tuberculosis Programme and the National Tuberculosis Reference Laboratory.
These activities include membership in the National TB-HIV Advisory
Committee, membership in the MDR TB Advisory Committee, regular meetings
with the National Tuberculosis Programme, a National TB Programme Review in
2006, revision of the 2007 National TB Programme manual, and support to
develop and conduct an evaluation of the national isoniazid preventive
therapy program in 2008.
Student Fellowship BOTUSA has a medical student fellowship to
provide third or fourth-year medical students the opportunity to participate
in CDC research in Botswana, as well as gain experience with clinical
medicine and culture in a developing country. For more information, visit
the American
Medical Student Association web site. |