Mark Connors, M.D.
Chief, HIV-Specific Immunity Section
Description of Research Program
The major focus of the HIV-Specific Immunity Section is identifying the mechanisms of an effective humoral and cellular immune response to HIV. Identifying the components, targets, and magnitude of such responses is a critical step toward the development of effective vaccines and immunotherapies. In our investigation, we recruit patients who are the best available examples of effective humoral or cellular responses to HIV and study their immune response in detail. The knowledge provided by this work offers fundamental insights about the regulation of human immune responses to HIV and other viruses.
Mechanisms of Immunologic Restriction of HIV Replication
(Stephen A. Migueles, M.D., Lead Investigator)
A number of recent studies have provided evidence for the role of CD8+ T cells in restricting lentivirus replication in vivo; however, the reason the CD8+ T-cell response fails to control HIV in the majority of infected individuals remains poorly understood. It is now clear that there exists a group of rare patients who maintain immunologic restriction of HIV replication, despite prolonged infection, without the use of antiretroviral therapy. Many of these patients have been infected for more than 20 years with no CD4+ T-cell decline. These patients, referred to as long-term nonprogressors (LTNP), or “elite controllers,” likely hold important clues to the components of an effective immune response to HIV.
Conversely, they also hold important clues regarding how control of HIV replication is lost in most infected individuals. We have now assembled a large cohort of patients with nonprogressive infection. Cells from these patients, as well as from major histocompatibility complex (MHC) matched and mismatched progressors as controls, are being used to systematically dissect the mechanisms of immune-mediated restriction of HIV replication.
Mechanisms of Broad Cross-Neutralization of HIV
(Nicole Doria-Rose, Ph.D., Lead Investigator)
There is a growing consensus that eliciting both neutralizing antibodies (NtAb) and a potent T-cell response will be necessary for an effective HIV vaccine. Eliciting NtAb to HIV, however, has been a challenging goal. Thus far, efforts to determine the mechanism(s) of broad cross-neutralization by patient sera have been hampered by a number of obstacles, including a lack of cohorts with broad responses, a lack of techniques to purify antigen-specific cells, and inefficient cloning techniques. Our work has overcome a number of these obstacles and is permitting a careful study of specificities that mediate broad cross-neutralization. Determining the specificities of these antibodies will likely be critical for the design of effective HIV vaccines.
Video
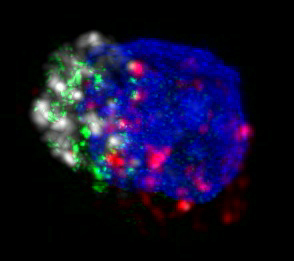 |
| HIV-specific killer T cell with perforin in green, granzyme B in white, MHC-tetramer in red, and nucleus in blue |
| View in Windows Media Format (mp2, 3.6 MB) |
Major Findings
- Within our cohort of LTNP is a dramatic overrepresentation of the B*5701 class I allele: 68 percent compared to 11 percent in the Caucasian U.S. population.
- CD8+ T-cell response to HIV-1 in LTNP is more narrow (typically directed toward three to four epitopes) than the response in progressors (typically 15 to 20 epitopes).
- CD8+ T-cell response of LTNP is highly focused on peptides restricted by the B*5701 allele, in most cases to the exclusion of peptides restricted by other alleles.
- Very high frequencies of circulating CD8+ T cells (0.8 to 18 percent IFN-g producing) were found to be HIV-specific in both LTNP and progressors, suggesting the difference in effectiveness of patients’ responses is qualitative rather than quantitative.
- The autologous virus is recognized by a high-frequency response in both patient groups. There are no differences between patient groups in the frequency of putative “escape mutations.”
- LTNP are distinguished by the maintenance of HIV-specific CD8+ T cells with a high proliferative capacity. This proliferation was found to be coupled to or parallel to perforin expression.
- HIV-specific CD4+ T cells in treated progressors are similar in frequency, function, and phenotype to those in LTNP and to non-HIV specific responses in uninfected controls.
- Previously described associations between viremia and changes in cytokine secretion or phenotype are an effect rather than a cause of loss of immunologic control of HIV.
Pictures
 |
| Members of the HIV-Specific Immunity Section 2008 |
 |
| Lab members conducting experiments |
 |
| Lab members at the AIDS Walk 2007 | |
 |
| Lab members discussing data | |
Selected Publications
To view a complete listing, visit PubMed.
Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Schwartz D, Sullivan J, and Connors M. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV infected long term nonprogressors. PNAS 2000;97(6);2709-2714.
Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan C, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, and Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunol 2002; 3(11); 1061-1068.
Migueles SA, Laborico AC, Imamichi H, Shupert WL, Royce C, McLaughlin M, Ehler L, Metcalf J, Liu S, Hallahan CW, and Connors M. The differential ability of HLA B*5701+ long-term nonprogressors and progressors to restrict HIV replication is not caused by loss of recognition of autologous viral gag sequences. J Virol 2003; 77(12); 6889-6898.
Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, Migueles SA, Laborico AC, Shupert WL, Hallahan CW, Davey RT Jr., Dybul M, Vogel S, Metcalf J, and Connors M. Diminished proliferation of HIV-specific CD4+ T Cells is associated with diminished IL-2 production and recovered by exogenous IL-2. J Virol 2003; 77(20); 10900-10909.
Migueles SA, Tilton JC, and Connors M. Qualitative host factors associated with immunologic control of HIV infection by CD8+ T cells. Curr Opin HIV AIDS 2005; 1; 28-33.
Tilton JC, Johnson AJ, Luskin MR, Manion MM, Yang J, Adelsberger JW, Lempicki RA, Hallahan CW, McLaughlin M, Mican JM, Metcalf JA, Iyasere C, and Connors M. Diminished production of monocyte proinflammatory cytokines during HIV viremia is mediated by type 1 interferons. J Virol 2006; 80; 11486-11497.
Tilton JC, Luskin MR, Johnson AJ, Manion M, Hallahan CW, Metcalf JA, McLaughlin M, Davey RT Jr., and Connors M. Changes in paracrine IL-2 requirement, CCR7 expression, frequency, and cytokine secretion, of HIV-specific CD4+ T cells are a consequence of antigen load. J Virol 2007; 81; 2713-2725.
Tilton JC, Manion MM, Luskin MR, Johnson AJ, Patamawenu AA, Hallahan CA, Cogliano-Shutta NA, Mican JM, Davey RT Jr., Kottilil S, Lifson JD, Metcalf JA, Lempicki RA, and Connors M. HIV Viremia Induces Plasmacytoid Dendritic Cell Activation In Vivo and Diminished Interferon-α Production In Vitro. J Virol 2008.
back to top