Spring 2008
NIAID Discovery News
Insights from People Who Keep HIV in Check Naturally
David first learned he had HIV in 1986. “I of course assumed that I’d be dead in two or three years at most,” he recalls. (This story uses a pseudonym to maintain his confidentiality.) His first sexual partner died of AIDS around 1993, and his second partner has been HIV-positive and on antiretroviral medications for many years. David used to refer to his regular 6-month medical check-up as his “lottery ticket,” never knowing when the virus would trigger disease in him as it had in so many others.
Twenty-two years after his diagnosis, David has not developed HIV-related disease despite never having taken medications to fight the virus. Remarkably, his immune system has remained healthy on its own.
Elite Controllers: Who They Are and What They Might Teach Us
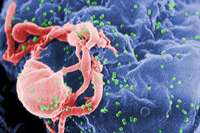 |
| Scanning electron micrograph of HIV emerging from a white blood cell. Green bumps on cell surface are sites of assembly and “budding” of virus particles. (Credit: CDC/C. Goldsmith, P. Feorino, E. L. Palmer, W. R. McManus) |
David is part of a small group of HIV-infected individuals—about 1 in every 3,000—whose immune systems naturally and durably control the virus. There is no standard definition for people like him, who are known variously as long-term nonprogressors, elite controllers, elite suppressors, or HIV controllers. Scientists generally define them as people who maintain no more than 50 copies of HIV per milliliter (mL) of blood over a period of at least 1 to 2 years despite never having taken antiviral medications. Some elite controllers have contained HIV at that low level for decades. In contrast, most HIV-infected individuals have between 10,000 and 1 million copies of HIV/mL before they start treatment. The higher the level of HIV, the more likely the person will develop disease and progress toward AIDS.
Elite controllers are of great medical interest. Their immune systems may inform the design of an HIV vaccine that suppresses virus replication and may reveal which cellular activities should be measured as markers of success in participants of HIV vaccine clinical trials.
Mark Connors, M.D., head of the HIV-Specific Immunity Section of NIAID’s Laboratory of Immunoregulation, has studied elite controllers for 15 years to unveil the clues their immune systems possess. He first identified these remarkable individuals during his work on virus-specific immunity in the early 1990s. Among participants in his study at that time, “there were a few people who had this inexplicable level of control of HIV,” he recalls. “We suspected that they were somehow different.” In experiments with specialized mice containing transplanted human cells where HIV replicates, Dr. Connors and his colleagues demonstrated that certain immune-system cells from the HIV controllers mediated control of the virus in the mice. That finding led him to focus on determining how controllers’ immune cells were keeping the virus at bay.
Since then, hundreds of scientists—many in collaboration with Dr. Connors—have attempted to uncover the mechanism or mechanisms behind HIV control. Most have concluded that biological characteristics of elite controllers, not a quirk of the virus, are responsible. But identifying the factors at work is an extremely complex task because the bodies of elite controllers appear to employ different combinations of tactics to suppress HIV.
Pursuing the Keys to HIV Control
Dr. Connors’ group focuses on immune-system cells known as CD8+ T cells, also called “killer” T cells. As their name implies, killer T cells hunt down infected cells and destroy them. Our bodies have billions of killer T cells, but only a relatively small number can identify any one particular disease-causing microbe.
To understand the unique characteristics of HIV-specific killer T cells in elite controllers, Dr. Connors, staff clinician Stephen Migueles, M.D., and their coworkers are comparing the immune functions of controllers with the immune functions of two groups of chronically HIV-infected people who cannot control the virus naturally—people known as “progressors” or “non-controllers.” One group of progressors has not started antiretroviral treatment, while the members of the other group take antiretroviral drugs that have reduced their viral loads to levels similar to those of controllers. All these study participants, including David and 49 other elite controllers, come to NIAID from around the country once every 6 months for medical checkups and to donate white blood cells and plasma, the liquid part of blood.
“It’s really gratifying to find a research study and people who… [are trying] to figure out the mechanism of what’s going on that allows me to be different,” says David, who learned about the study through an online chat on an AIDS-focused Web site. (Volunteers continue to be recruited.)
Controllers and untreated progressors initially have comparable proportions of HIV-specific killer T cells relative to the total number of T cells in their blood. Yet in the controllers Drs. Connors and Migueles have studied, HIV-specific killer T cells have unique qualities that enable them to destroy HIV-infected cells.
For instance, in controllers, once an HIV-specific killer T cell detects an HIV-infected cell, the killer T cells quickly multiply. In progressors, however, they don’t. In fact, controllers have 20 times more HIV-specific killer T cells than treated progressors.
Also, in the presence of HIV, controllers’ HIV-specific killer T cells boost production of a molecule called perforin that pokes holes in HIV-infected cells, creating entryways for other molecules that program the infected cells to die. This boost in perforin production doesn’t happen in progressors.
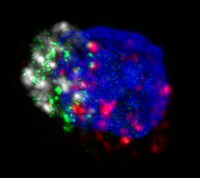 |
| HIV-specific killer T cell with perforin in green. In the presence of HIV, elite controllers’ HIV-specific killer T cells boost production of perforin, which pokes holes in HIV-infected cells, creating entryways for other molecules that program the infected cells to die. (Credit: NIAID/Owen Schwartz and Jurai Kabat) |
Furthermore, when molecules on the surface of HIV-infected CD4+ T cells—the chief immune-system cells that HIV uses to replicate itself—present pieces of chopped-up viral proteins to the immune system, the HIV-specific killer T cells of most controllers home in on a limited number of protein fragments presented by just one molecule. In contrast, progressors’ HIV-specific killer T cells respond to 15 to 20 different protein fragments presented by a range of molecules on CD4+ cells, yet more is not better.
In addition, the killer T cells of controllers tend to be better “multitaskers” than those of progressors because they send out a greater variety of chemical messengers in larger quantities. This characteristic is called polyfunctionality.
In collaboration with Dr. Connors’ group, Richard Koup, M.D., of the immunology laboratory in NIAID’s Vaccine Research Center, identified polyfunctionality in both killer T cells and CD4+ T cells. Interestingly, Dr. Koup has found polyfunctionality to be a common thread across multiple viral infections controlled by the human immune system. “The more polyfunctional your T cells, the better the virus is controlled,” he says, “or the better the virus is controlled, the more polyfunctional your T cells.” More research is needed to determine whether polyfunctionality is a cause or an effect of low viral levels.
Dr. Connors is also investigating cause and effect of the proliferation of killer T cells and their accelerated production of perforin—normal biological activities of healthy immune systems in response to a pathogen. Dr. Connors’ group has demonstrated that using HIV medications to lower progressors’ virus levels to the same point as that of elite controllers does not stimulate HIV-specific killer T cells to proliferate or to make more perforin. This indicates that progressors’ HIV-specific killer T cells have a defect that persists even in the presence of low levels of HIV. But proving that the proliferation of HIV-specific killer T cells and their ramped-up production of perforin are actually driving forces behind HIV control will require more research. Dr. Connors says his group is gradually beginning to develop animal models to test this hypothesis. If he can prove causation in an animal, his work will suggest that stimulating killer T cells to proliferate and boost production of perforin may be part of the recipe for a vaccine or therapeutic that would help some people maintain low levels of HIV.
Many elite controllers who volunteer in studies like those of Dr. Connors relish the opportunity to contribute to scientific understanding of HIV and AIDS. “It’s one of the most amazing things I’ve ever been involved with,” says David, who enrolled in the NIAID study in 2006. “I wish that I’d gotten involved earlier.” Some elite controllers are willing to go so far as having a spinal tap or a gut-tissue biopsy to help HIV/AIDS researchers investigate unanswered questions. As far as David is concerned, “I’m just really glad there are people…who are willing to dedicate their lives to doing this research.”
—Laura Sivitz
References
MR Betts et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107(12):4781–89 (2006).
SA Migueles and M Connors. The role of CD4(+) and CD8(+) T cells in controlling HIV infection. Current Infectious Disease Reports 4(5):461–67 (2002).
SA Migueles et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature Immunology 3(11):1061–68 (2002).
SA Migueles et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV infected long term nonprogressors. Proceedings of the National Academy of Sciences 97(6):2709–14 (2000).
back to top