Researchers Seek Novel Methods to Stop the Transmission of Sleeping Sickness
NIAID-funded scientists in Uganda are working on an innovative research initiative to stop the transmission of Human African Trypanosomiasis (HAT), also known as sleeping sickness. HAT afflicts tens of thousands of people in sub-Saharan Africa and leads to fever, swollen lymph nodes, and insomnia or excessive amounts of sleep. If left untreated, HAT can be fatal.
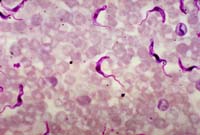 |
A micrograph of a rat blood smear revealing Trypanosoma brucei parasites. Infected tsetse flies spread trypanosome parasites to humans, causing African trypanosomiasis, or sleeping sickness.
Credit: CDC/Dr. Mae Melvin |
HAT is caused by the trypanosome parasite, which resides in the tsetse fly. Tsetse flies transmit the parasite, and therefore the disease, to humans while taking a blood meal. Scientists propose to genetically alter the tsetse fly population so that it is resistant to the trypanosome parasite and stops spreading HAT to humans.
Tsetse flies inherit several bacteria from their mothers that can be instrumental in stopping the transmission of HAT to humans. One of these bacteria, called Sodalis, is found in several areas of the tsetse, including the gut and sometimes the salivary glands. Because the trypanosome parasite develops in the gut tissue, the stomach tissue, and the salivary glands of the tsetse, Sodalis bacteria are in a prime position to affect the parasite’s growth and transmission. Scientists hope to alter Sodalis so that it contains anti-trypanosome genes. They would then inject the genetically modified bacteria into female tsetse flies, and the offspring of these flies would be resistant to the trypanosome parasite.
To successfully interfere with HAT transmission to humans, the trypanosome-resistant flies would have to become the dominant flies in the tsetse population. Scientists plan to make this happen by using another naturally occurring bacterium in tsetse flies called Wolbachia. Wolbachia is found in only some tsetse flies, but those flies spread quickly because of a unique advantage they have in the reproductive process: Females infected with Wolbachia are able to mate with males infected with Wolbachia and with males who are not; non-infected females are able to mate only with non-infected males. As a result, Wolbachia-infected females are able to produce more offspring than their non-infected counterparts, and the infected females pass the bacterium to all their offspring.
Scientists hope to use this Wolbachia advantage to replace the natural tsetse population with the genetically altered, trypanosome-resistant tsetse. They would inject the trypanosome-resistant Sodalis into females infected with Wolbachia and then release their progeny into the natural tsetse population. Because of their reproductive advantage, the genetically altered flies would eventually overtake the natural tsetse, resulting in a population that would be resistant to the trypanosome parasite.
The NIAID-supported study will involve a combination of laboratory and field experiments that will help scientists prepare for this replacement strategy. Researchers will study the basic genetic structure and biology of the tsetse, the biology of Sodalis and Wolbachia, and the links between the two bacteria. Using this knowledge, they will create a model framework that will allow them to design and predict the success of the replacement strategy. Better understanding the tsetse may also allow scientists to discover new opportunities for intervention and to support more traditional control strategies.
back to top