New Ammunition against an Old Enemy: Multiple Approaches to TB Therapy
Searching for new drugs against tuberculosis can be quite frustrating, NIAID's Clifton Barry, III, Ph.D., admits. It is a simple matter to destroy Mycobacterium tuberculosis (M. tb), the microbe that causes tuberculosis, in a test tube. But M. tb has an extraordinary ability to live in a state of nearly suspended animation within immune system cells, so completely ridding the body of M. tb is difficult.
Still, Dr. Barry believes that many of the two million deaths each year from TB could be prevented with better drug therapies. He and his collaborators have used two techniques, one a "kitchen sink" approach, the other involving repeated chemical tweaking, to screen chemicals for their ability to kill M. tb.
Kitchen Sink
The kitchen sink approach began with an established anti-TB drug called ethambutol. Dr. Barry's team developed a way to easily create thousands of compounds that differ only slightly from ethambutol. More than 100,000 of these compounds were screened in Dr. Barry's lab, the idea being that from such a vast number some were bound to prove more active than the original. Indeed, several hundred of the compounds deserved a closer look.
Marina Protopopova, Ph.D., who participated in the original screening project in Dr. Barry's lab, continued winnowing the promising candidates with her colleagues at Sequella, Inc., in Rockville, MD. This work is supported by NIAID's challenge grant program which encourages private-sector involvement in tackling the world's most persistent infectious diseases. The research was also aided by an inter-institute program involving NIAID and the National Cancer Institute, says Dr. Protopopova.
 |
|
Dr. Protopopova (back row, left) and her colleagues
Credit: Marina Protopopova, Ph.D. |
Starting with 70 of the most potent compounds, which could both kill the whole TB organism and attack its cell wall, Dr. Protopopova and her coworkers whittled down the number to 26. Next, those 26 compounds were tested for cytotoxicity and activity against the bacteria in macrophages to determine if they had the right attributes to become drugs. Six that did were then tested in mice.
One candidate, chemically quite different from the original drug, emerged with strengths that surprised even the researchers who made it. Code named SQ-109, the drug quickly travels to the lungs—where most TB bugs reside—and kills the bacteria instead of merely stopping their growth, as do some other TB drugs. Moreover, SQ-109 is proven to be highly effective against multidrug-resistant TB (MDR-TB). The Sequella group's findings from mouse studies showed that a combination of SQ-109 and the first-line TB drug rifampicin yielded enhanced anti-TB activity that continued to work for some time after the drug therapy ended.
In March 2006, Sequella announced that it received a worldwide license from the National Institutes of Health to continue development of SQ-109. The company says it plans to begin Phase I human clinical trials of the experimental drug during 2006.
Chemical Tweaks
The chemical tweak approach started with a compound originally tested for cancer-fighting properties. William Baker, Ph.D., a chemist at a Seattle biotech company, found mention of this failed cancer drug in a search of scientific literature. He developed more than 300 chemical variations of the starting compound and tested each one for anti-TB activity in both test tubes and mice.
Dr. Barry and others collaborated with Dr. Baker and with C. Ken Stover, Ph.D., to learn exactly how these related chemicals work against M. tb's tough cell wall. Time and again, the scientists made tiny adjustments in the atomic arrangements of the compounds, hoping with each tweak to make the substance better at destroying the bacteria. At last, one candidate emerged that stood out from the others.
This compound, known as PA-824, works well not only against actively dividing M. tb, but seems to attack the bacteria in its resting state as well. If true, PA-824 might shorten the length of time typically needed to treat TB. The investigators published their initial findings in June 2000.
In February 2002, Chiron Corporation, the drug company that owns the patent on PA-824, entered an agreement with the nonprofit organization Global Alliance for TB Drug Development to continue developing the potential drug. Extensive testing in animal models of disease showed that the drug was well absorbed and traveled rapidly to key organs, such as the lung and spleen. Under the sponsorship of the Global Alliance, PA-824 entered the first phase of human clinical trials in June 2005.
Dr. Barry's group at NIAID and the Novartis Institute for Tropical Diseases in Singapore (a nonprofit research foundation set up by Novartis Pharmaceuticals to address drug development for diseases of the developing world) have also entered into a partnership to further refine PA-824 by working on a “back-up” series to provide a chemical safety net in case the main contender fails in early clinical trials. This strategy relies on making additional chemical analogs of this series of molecules and trying to work around any potential pitfalls encountered as PA-824 is tested in humans for the first time.
Dr. Barry's lab is also involved in research to predict which tweaks will work before they are actually made. Combining computer modeling and X-ray crystallography with chemical synthesis, the scientists can maximize their chances of success in producing highly effective drug candidates. Together with scientists from the St. Jude Children's Hospital in Memphis, Dr. Barry and his coworkers are improving a potent antibacterial substance derived from a soil-dwelling microbe. The substance, thiolactomycin, shows potential to treat TB as well as common bacterial infections that have developed resistance to other antibiotics.
"Developing drugs for a neglected disease like TB has not been a priority for most pharmaceutical companies," Dr. Barry notes. "It costs at least $300 million to take a drug candidate from lab to clinic. Drug companies have to be convinced that the market for a new drug is large enough to warrant that kind of spending. We're piggybacking the work on thiolactomycin as an anti-TB drug onto its development as a potential treatment for more common infections to give the companies more bang for their buck," he adds.
Early Promise against Latent TB
At FASgen, a biotech company in Baltimore, scientists are developing a TB drug that cripples the ability of M. tb to build up its waxy outer coating. The compound, FAS20013, has a number of characteristics that make it a promising drug candidate, says company CEO Albert H. Owens, Jr., M.D. For example, he notes, it can be synthesized in large quantities inexpensively and in a relatively simple three-step process. FAS20013 is readily absorbed when given orally to mice, which is the preferred method of delivering drugs to people as well. Most importantly, FAS20013 appears in preliminary testing to be potent against both MDR-TB and against TB bacteria that are not actively dividing.
FAS20013 works differently than any other currently used TB drug, says Dr. Owens. Not only does it inhibit the biosynthesis of M. tb’s cell wall, it also interferes with the bacterium’s energy-generating metabolic pathways, he says. In experiments so far, extremely low concentrations of FAS20013 were able to kill M. tb bacteria, yet human cells were not harmed even when exposed to high levels of the compound. One explanation for this characteristic may be that FAS20013 targets energy-generating pathways that are not structurally homologous to those in human metabolism, notes Dr. Owens.
If further testing in test tubes and in animals goes well, Dr. Owens says the company hopes to begin Phase I clinical trials in humans before the end of 2006. The first trials would focus on the compound’s safety and its potential to fight MDR-TB, he adds.
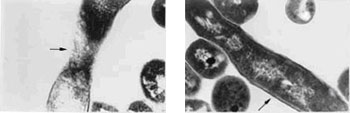 |
Electron micrographs of TB bacteria shown in cross-section. The arrows point to the normally thick wall of a virulent organism (right) and a treated organism (left). The treated organism’s cell wall has been eroded as one of the FASgen compounds has interfered with its biosynthesis.
Credit: FASgen, Inc. |
Related Links
Government
Non-government
back to top