|
New approaches to HIV prevention are urgently needed to stem the estimated 2.7 million new HIV infections that occur
worldwide each year. While behavior change programs have contributed to dramatic reductions in the number of annual
infections in the U.S. and many other nations, far too many individuals remain at high risk. With an effective vaccine years
away, there is mounting evidence that antiretroviral drugs may be able to play an important role in reducing the risk of
HIV infection. As part of its commitment to developing new HIV prevention strategies, the Centers for Disease Control
and Prevention (CDC) is sponsoring three clinical trials of pre-exposure prophylaxis, or PrEP, for HIV prevention, and is
participating in a University of Washington-sponsored trial in Kenya and Uganda. The trials test the antiretroviral drug
tenofovir disoproxil fumarate (or tenofovir, brand name Viread®) used alone or in combination with emtricitabine (together,
known as the brand name Truvada®) taken as a preventative drug.
The CDC-sponsored trials are designed to answer important questions about the safety and efficacy of a tenofovir or tenofovir
plus emtricitabine pill taken as a daily oral HIV preventative among three populations at high risk for infection: heterosexuals
in Botswana, injection drug users in Thailand, and men who have sex with men (MSM) in the United States. The trials in
Botswana and Thailand are safety and efficacy trials, while the U.S. trial is an extended safety trial. CDC will also co-manage
two trial sites in Uganda as part of the University of Washington Partners PrEP Study, which is examining the safety and
efficacy of PrEP among heterosexual couples. All of the trials will also assess the effects of taking a daily pill on HIV risk
behaviors, adherence to and acceptability of the regimen, and in cases where participants become HIV-infected, the resistance
characteristics of the acquired virus. This information will be critical to guide future studies and HIV prevention programs.
Similar PrEP trials are also being conducted by other agencies. In 2006, Family Health International (FHI), with funding from
the Bill and Melinda Gates Foundation, completed a safety trial of tenofovir for HIV prevention among young women in
Ghana. The study provided the first data showing PrEP with tenofovir to be both safe and acceptable for use by HIV-negative
individuals. The National Institutes of Health (NIH) is currently evaluating the safety and efficacy of PrEP among MSM in Peru,
Ecuador, South Africa, and the U.S. and plans to expand its trial to additional countries. Additional trials investigating PrEP
among women in Africa are scheduled to be launched by FHI and the Microbicide Trials Network (MTN) within the next year.
Rationale for Trials of Pre-Exposure
Prophylaxis for HIV Prevention
Researchers believe that an
antiretroviral drug taken as a daily oral
preventative is one of the most important
new prevention approaches being investigated
today. An effective daily preventative
treatment could help address the urgent need
for female-controlled prevention methods
and, when combined with existing prevention
measures, could help reduce new HIV
infections among men and women at high risk.
Characteristics of Current PrEP Candidates
- Established safety as HIV
treatments
- Potent antiretrovirals
- Long duration of action
- Once-daily dosing
- Low levels of resistance
|
|
The concept of providing a preventative treatment before exposure to an
infectious agent is not new. For example, when individuals travel to an area
where malaria is common, they are advised to take medication to fight malaria
before and during travel to that region. The medicine to prevent illness will
then be in their bloodstream if they are exposed to the infectious agent that
causes malaria.
Several sources of data suggest that the use of antiretroviral drugs in this manner
may be effective in reducing the risk of HIV infection. Theoretically, if HIV
replication can be inhibited from the very first moment the virus enters the body,
it may not be able to establish a permanent infection. Providing antiretrovirals
(ARVs) to HIV-infected women during labor and delivery and to their newborns
immediately following birth has been shown to reduce the risk of mother-to-child
transmission by about 50 percent. Additionally, in observational studies, ARV
regimens have been associated with an 80 percent reduction in the risk of HIV
infection among health care workers following needle sticks and other accidental
exposures, when treatment is initiated promptly and continued for several weeks.
Finally, animal studies have shown that tenofovir can reduce the transmission
of a virus similar to HIV in monkeys when given before and immediately after
a single retroviral exposure. Animal studies have also demonstrated that preexposure
administration of tenofovir plus emtricitabine provided significant
protection to monkeys exposed repeatedly to an HIV-like virus. These data, combined with the drugs’ favorable resistance
and safety profiles as HIV treatments, make tenofovir and tenofovir plus emtricitabine ideal candidates for HIV
prevention trials.
Tenofovir was approved by the U.S. Food and Drug Administration in 2001 as a treatment for HIV infection, and the
tenofovir plus emtricitabine combination pill was approved for use as an HIV treatment in 2004. More than 200,000 HIVinfected
people around the world have now used these drugs. As treatments for HIV-infected individuals, tenofovir and
tenofovir plus emtricitabine have been shown to be both safe and effective. They have relatively low levels of side effects
and slow development of associated drug resistance, compared with other available HIV treatments. Because the therapies
are taken orally only once a day, with or without food, they are also among the most convenient-to-use HIV drugs available
today. These trials are designed to evaluate the drugs’ safety and efficacy among uninfected individuals. Side effects may
differ in HIV-negative populations, and it is not yet known if tenofovir or tenofovir plus emtricitabine can prevent HIV
infection in humans.
CDC-Sponsored PrEP Trials
Specific Trial Designs and Objectives
All three of CDC’s studies are randomized, double-blind, placebo-controlled trials. All participants receive
risk-reduction counseling and other prevention services. In addition, half of the participants are randomly assigned to
receive one antiretroviral pill daily (either tenofovir or tenofovir plus emtricitabine, depending on the trial), and the other
half are randomly assigned to take one daily placebo pill (a similar tablet without active medication). Neither researchers
nor participants know an individual’s group assignment. In all, the studies will involve 4,000 volunteers. The Thailand and
U.S. trials of tenofovir began in 2005 and the Botswana trial of tenofovir plus emtricitabine began in early 2007. The trials
are expected to last between four and six years.
To ensure that the studies remain on a solid scientific and ethical foundation, all study procedures and plans are reviewed
and approved by scientific and ethical review committees at CDC (called institutional review boards, or IRBs), as well
as IRBs established by each host country and research site prior to trial launch. Additionally, data on safety, enrollment,
and efficacy will be reviewed regularly by an independent data safety and monitoring board (DSMB) for the Botswana
and Thai trials, and by an independent safety review committee for the U.S. trial. These committees review emerging
data to ensure that continuing the trial is safe and to determine the point at which the results are conclusive. If scientific
questions arise during the course of the research, these committees will meet more frequently.
Botswana and Thailand
The CDC trials in Botswana and Thailand are safety and efficacy trials. The Botswana trial is examining the safety and
efficacy of tenofovir plus emtricitabine, and the Thailand trial is examining the safety and efficacy of tenofovir.
 |
 |
-
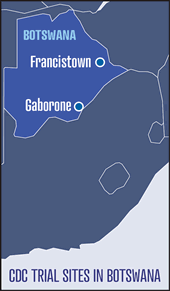
Botswana — The Botswana study
is being conducted in collaboration
with the Botswana government and is
enrolling 1,200 HIV-negative heterosexual
men and women, ages 18 to 39, in the
nation’s two largest cities, Gaborone and
Francistown. Participants are recruited
through a number of venues, including
HIV voluntary counseling and testing
centers, sexually transmitted disease
(STD) and family planning clinics, youth
organizations, and community events.
-
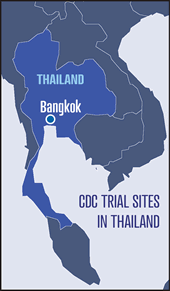
Thailand — The Thailand study is
being conducted in collaboration with the
Bangkok Metropolitan Administration and
the Thailand Ministry of Public Health
and is enrolling 2,400 HIV-negative intravenous drug users (IDUs) at 17 drug
treatment clinics in Bangkok. Participants are recruited at the drug treatment
clinics, at community outreach sites, and through a peer referral program.
The United States
The U.S. trial is designed to assess the clinical and behavioral safety of once-daily tenofovir among
HIV-negative MSM. This trial will not be large enough to evaluate the drug’s efficacy in reducing HIV transmission. Data
from the CDC trial will provide critical information to guide the development of guidelines for use, should efficacy be
demonstrated in other trials.
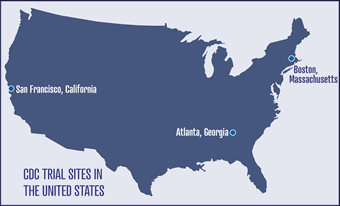
- United States — The U.S. study
is being conducted at three sites in
collaboration with the San Francisco
Department of Public Health, the AIDS
Research consortium of Atlanta, and
Fenway Community Health in Boston.
The study has enrolled 400 HIV-negative MSM who reported having had anal
intercourse in the prior 12 months.
Participants are randomly assigned to
one of four study arms. Two arms receive
either tenofovir or placebo immediately
upon enrollment, while the other two
arms receive either tenofovir or placebo after nine months of enrollment. This design will allow researchers to compare risk
behaviors among those taking a daily pill and those not taking pills.
Education and Enrollment of Trial Participants
Understanding the potential impact of a daily preventative drug regimen on HIV risk behaviors will be critical, should
pre-exposure prophylaxis prove effective in reducing HIV transmission. One of the greatest risks, as efforts progress to
identify new biomedical prevention approaches, is that individuals at risk will reduce their use of existing HIV prevention
strategies. It will therefore be crucial to reinforce proven behavioral prevention strategies, both within and beyond
these trials. All three trials are taking multiple steps to address this issue during the education and enrollment of trial
participants and through ongoing participant counseling.
First, it is critical to ensure that participants understand that trial participation may not protect them from HIV
infection—either because they may receive a placebo or because they may receive a study drug, the efficacy of which
remains unproven. This and other key aspects of the trial, including the potential risks and benefits of participation, are
explained to potential volunteers in the language of their choice, prior to their enrollment. To ensure participants fully
understand all aspects of their participation, all volunteers are required to pass a comprehension test prior to providing
written informed consent. Study participants are also free to withdraw from the trial at any time and for any reason.
Risk-Reduction Counseling and
Other Prevention and Treatment Services
To assist participants in eliminating or reducing HIV risk behaviors, extensive counseling is provided at each study visit, and
more often if needed. This interactive counseling has proven effective in reducing the risk of HIV and other STDs in multiple
populations, including past participants of similar HIV prevention trials. Participants are also offered free condoms and STD
testing and treatment to reduce their risk for HIV infection. Additionally, in Thailand, participating IDUs
are offered follow-up in a methadone drug
treatment program and receive bleach and
instructions on how to use it to clean
needles. Consistent with Thai government
policy, sterile syringes are not provided,
but are widely available in Thailand without
a prescription and at low cost (one sterile
syringe and one needle cost about 5 baht, or
about $0.15).
While participants will likely be at lower risk as a result of these prevention services, some individuals
will engage in behavior that places them at risk for HIV infection. To ensure that participants who are
infected during the trial are quickly referred to the best available medical and psychosocial services,
participants receive free rapid HIV testing at every visit. This regular HIV testing will also help guard against the
development of drug-resistant virus, as the study drug will be immediately discontinued when infection is detected.
Participants who become infected receive confirmatory testing for infection, post-test risk-reduction and support
counseling, and help enrolling in local HIV care programs. Both Thailand and Botswana have antiretroviral treatment and
HIV care programs in place at minimal or no cost to patients. In the United States, participants are referred to local health
care providers or public health programs for needed medical and social services.
Additionally, to help guide treatment decisions and to determine if prior exposure to tenofovir or tenofovir plus
emtricitabine has any effect on the course of disease, initial testing will be provided for viral load, CD4 count, and HIV
resistance mutations. Participants will also be followed for an additional 12 months following infection to examine their
immune and virologic response. Although study procedures ensure a very low risk of drug-resistant virus emerging, the
initial HIV resistance testing will provide important data on the degree to which any resistance does occur.
Monitoring for Side Effects
The health of participants is closely monitored throughout each trial, and participants are linked to any necessary
medical care. In addition to scheduled reviews of safety data by the DSMB, both clinical and behavioral safety are closely
monitored on an ongoing basis.
Although the drugs being tested have excellent safety profiles, there are potential medical risks. Tenofovir has been
associated with minor side effects such as nausea, vomiting, and loss of appetite, as well as rare but more serious
effects, such as impaired kidney function or reductions in bone density. Tenofovir plus emtricitabine has similarly been
associated with a relatively low level of side effects, including diarrhea, nausea, fatigue, headache, dizziness, and rash,
with infrequent reports of more serious side effects, such as impaired kidney function and lactic acidosis (a build-up of
lactic acid in the blood). For both drugs, these effects have largely been reversed after use of the drug was discontinued.
Careful monitoring is provided using laboratory testing for any biological abnormalities (such as elevated creatinine or
decreased phosphorus), so that the drug being tested can be promptly discontinued if serious concerns are identified. CDC will work with partners in each community to ensure that care is provided if either drug results in any health
problems during the trial.
Community Involvement
CDC has and will continue to work closely with community partners at each research site to ensure active community
participation during the planning and implementation of these trials.
- Botswana — In Botswana, community advisory boards have been established at each site, which include
representatives from local governments (elected and traditional), as well as community members and representatives
from key stakeholder organizations. Participant advisory boards have also been established. These groups provide input to
researchers throughout the trial.
- Thailand — In Thailand, a community relations committee, composed of injecting drug users from each of the 17 drug
treatment centers, family members, and representatives of local community organizations, meets regularly and provides
advice to study staff on all aspects of study design, implementation, and trial conduct.
- United States — In the United States, all three sites have established active community advisory boards
that are consulted regularly about study procedures and educational materials for potential participants.
Members of these boards will provide ongoing advice throughout the trials.
In addition to the regular input received by these established committees, broader outreach and consultations with
advocates and community-based organizations representing populations at risk for HIV are being held, as needed, to
address current and future plans for HIV prevention research and programs.
CDC Participation in Partners PrEP Study
The University of Washington is working with collaborators in Kenya and Uganda to conduct the Partners PrEP Study, which
is examining the safety and efficacy of two different PrEP regimens — once-daily tenofovir and once-daily tenofovir plus
emtricitabine — among heterosexual couples. CDC will co-manage two trial sites in Uganda, in conjunction with The AIDS
Support Organization (TASO), the largest indigenous non-governmental organization providing HIV care in Uganda.
This randomized, double-blind, placebo-controlled study will operate at eight trial sites in Kenya and Uganda and include 3,900 serodiscordant couples (couples in which one person is HIV-infected and the other is not). Stable serodiscordant couples are
the largest risk group for HIV infection in Africa, and this trial will provide important data on whether PrEP could be used to
prevent new HIV infections among this population. HIV-uninfected partners will be assigned to three groups: one group will
receive tenofovir, a second group will receive tenofovir plus emtracitabine, and the third group will receive a placebo. All
participants will receive ongoing risk reduction counseling and HIV testing, and their safety will be monitored by the
study’s DSMB and local IRBs. HIV-infected members of the discordant couples will receive ongoing HIV care.
The trial is the first to test the safety and efficacy of both tenofovir and tenofovir plus emtricitabine in the same population
and will allow investigators to simultaneously evaluate the two drugs as candidates for use as PrEP.
Planning for Possible Implementation of PrEP
As we move forward with the search for new HIV prevention strategies, it will be critical to determine how these
approaches can best be integrated into existing programs, should they prove effective in reducing risk. Because no
strategy is 100 percent effective in preventing HIV infection, the future impact of PrEP will ultimately be determined by
how effectively strategies are used in combination to provide the greatest protection to individuals at risk.
CDC has begun to examine potential implementation strategies with a wide range of stakeholders in the U.S. Experts are
examining a number of critical issues including possible funding streams, risk assessment tools, and delivery of PrEP as
part of a comprehensive prevention program. Additionally, CDC is conducting research to determine effective models for
reaching the populations at greatest risk in the U.S.
At the international level, should efficacy be proven, WHO and UNAIDS would develop normative guidance on PrEP
implementation, and individual countries would develop their own programs and policies for integrating PrEP into
prevention efforts. As these plans are developed, CDC will provide technical assistance to its international partners and
to local countries where CDC trials are being conducted.
|