|
Rocky Mountain Spotted Fever Home > Laboratory Detection
Laboratory Detection
Although it is technically feasible, specific rapid
laboratory confirmation of early Rocky Mountain spotted
fever is rarely done. Therefore, treatment
decisions should be based on epidemiologic and clinical
clues, and should never be delayed while waiting for
confirmation by laboratory results. Fundamental
understanding of the signs, symptoms, and epidemiology
of the disease is crucial in guiding requests
for tests for Rocky Mountain spotted fever, sample collection
and submission, and interpretation of laboratory results.
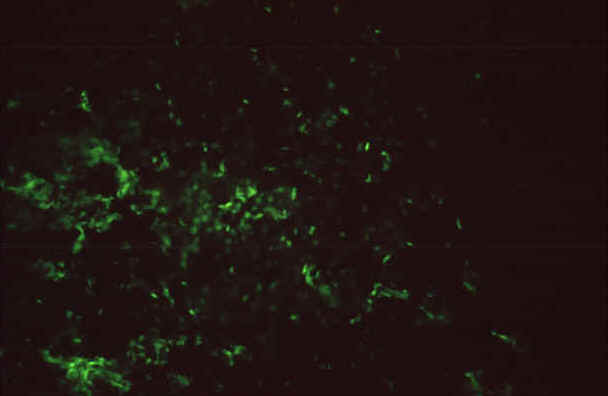 |
| IFA reaction
of a positive human serum on Rickettsia rickettsii
grown in chicken yolk sacs, 400X |
Routine clinical laboratory findings suggestive of
Rocky Mountain spotted fever may include abnormal
white blood cell count, thrombocytopenia, hyponatremia,
or elevated liver enzyme levels (see
Glossary for definitions of terms). Serologic
assays are the most widely available and frequently
used methods for confirming cases of Rocky Mountain
spotted fever. The indirect
immunofluorescence assay (IFA) (see figure) is generally
considered the reference standard in Rocky Mountain
spotted fever serology and is the test currently used
by CDC and most state public health laboratories, but
other well validated assays including ELISA, latex agglutination,
and dot immunoassays can be used.
IFA can be used to detect either IgG or IgM antibodies.
Blood samples taken early (acute) and late (convalescent)
in the disease are the preferred specimens for evaluation.
Most patients demonstrate increased IgM titers by the
end of the first week of illness. Diagnostic levels
of IgG antibody generally do not appear until 7-10 days
after the onset of illness. It is important to
consider the amount of time it takes for antibodies
to appear when ordering laboratory tests, especially
because most patients visit their physician relatively
early in the course of the illness, before diagnostic
antibody levels may be present. The value of testing
two sequential serum or plasma samples together to show
a rising antibody level is very important in confirming
acute infection with rickettsial agents because antibody
titers may persist in some individuals for years after
the original exposure to any of a number rickettsial
agents. IgG antibodies are more specific and reliable
since other bacterial infections can also cause elevations
in riskettsial IgM antibody titers.
The most rapid and specific diagnostic assays for Rocky
Mountain spotted fever rely on molecular methods like
PCR which can detect DNA present in 5-10 rickettsiae
in a sample. While organisms can be detected in whole
blood samples obtained at the acute onset of illness
in a few hours, rickettsial DNA is most readily detected
in fresh skin biopsies like those used in immunostaining
procedures. PCR can also be done on the fixed tissues
used in immunostaining, but it is less sensitive than
with unfixed tissues. PCR methods can be R. rickettsii-specific
but are usually confirmed by DNA sequencing of the amplified
gene fragments. Consequently, this procedure is more
specific than antibody-based methods which are often
only genus or spotted fever group-specific. Specified
diagnosis can also be confirmed by isolation of R.
rickettsii from clinical samples like whole blood
and biopsies. Materials can be shipped unfrozen or frozen
and on dry ice to ensure optimal chances of isolation
at the CDC. Isolation may require several weeks, but
isolates are very important for investigating differences
in the pathogenic properties and antimicrobial resistance
of rickettsiae which cause disease in different parts
of the United States.
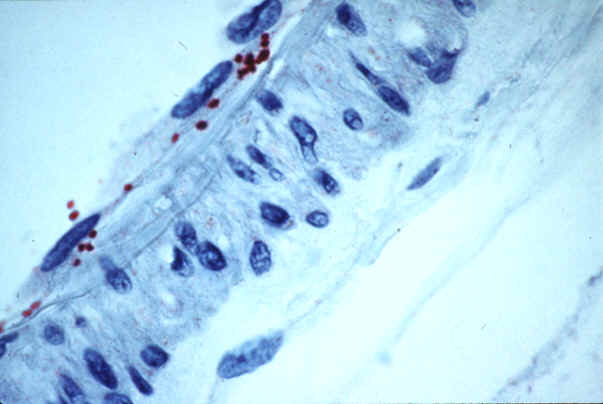 |
| Rickettsia
rickettsii in endothelial cells of a blood vessel
from a patient with fatal RMSF |
Another approach to Rocky Mountain spotted fever diagnostics
is
immunostaining. This method is used by taking a
skin biopsy of the rash from an infected patient prior
to therapy or within the first 48 hours after antibiotic
therapy has been started. Because rickettsiae
are focally distributed in lesions of Rocky Mountain
spotted fever, this test may not always detect the agent.
Even in laboratories with expertise in performing this
test, the sensitivity is only about 70% on biopsied
tissues because of the scarcity of organisms in some
samples. This assay may also be used to test tissues
obtained at autopsy and has been used to confirm Rocky
Mountain spotted fever in otherwise unexplained deaths
(see figure). Immunostaining for spotted fever
group rickettsiae is offered by the CDC, a few state
health departments, and some university-based hospitals
and commercial laboratories in the United States.
Date last reviewed: 05/20/2005 |