CBER Presentation
UK experience regarding malaria antibody tests and their contribution to blood safety
FDA Workshop on Testing for Malarial Infections in Blood Donors
July 12, 2006
Professor PL Chiodini
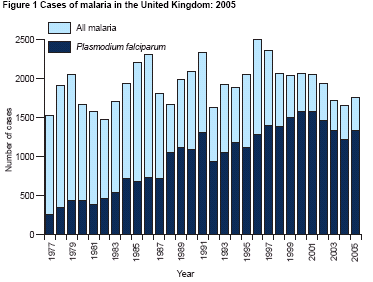
UK Malaria 2005
- Plasmodium falciparum 1338
- Plasmodium vivax 258
- Plasmodium ovale 116
- Plasmodium malariae 29
- Mixed infections 10
- Unspecified 3
- TOTAL 1754
UK MRL Data 2005
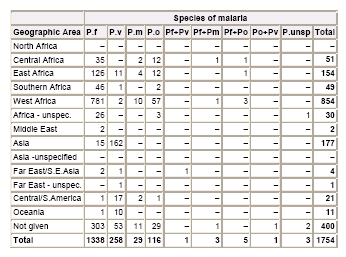
UK MRL Data 2005
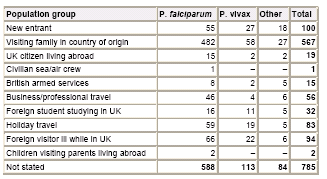
UK MRL Data 2004
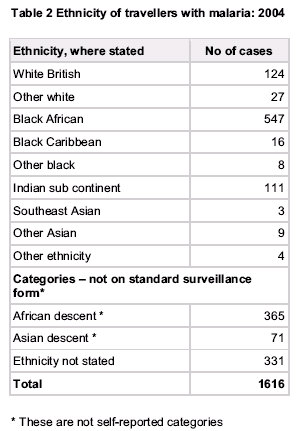
Risk Groups for TTM
Travellers:
- No, or almost no, immunity to malaria
- Almost always symptomatic if parasites present; thus excluded from donation
- Almost all P.falciparum in this group occurs in the first 2 months; virtually none after 6m
"Residents"
- Almost all brought up in sub-Saharan Africa
- Still partially immune to malaria disease
- May be asymptomatic but parasitaemic
- May harbour P.falciparum for years
TTM in the UK 1986-2006
5 cases, all due to P.falciparum. Donors:
- Semi-immune European, 10y in Africa. History of travel not given. Blood film negative. IFAT strongly positive
- West African. Blood collected for plasma usage, but inadvertently issued as whole blood after 19 days' storage at 4C in CPDA-1. IFAT strongly positive
- West African. Donated 2 months after a visit there. Travel history not elicited. IFAT and ELISA positive.
- African. Donated 2y11m after travel. Exclusion period at that time was 3y. ELISA & IFAT +ve
- West African. Last visited 8 years previously. Exclusion period at that time was 5y. IFAT and ELISA positive. PCR positive.
Thus, UK Risk Factors for TTM are:
- Failure of history taking
- Administrative error (less likely now)
- "Semi-immune" individuals; both residents AND long-term expatriates
Strategies for Prevention of TTM
- Complete prevention may not be possible with current methodology
- Aim to minimise the risk of introducing malaria parasites into the blood supply, without excluding potential donors unnecessarily
Identifying Potential Malaria Risk in a Donor
- History taking
- Time exclusion
- Screening donor serum for antimalarial antibodies
History
- Geographical location
- Length of time resident; as adult or child
- Time elapsed since there
- History of past malaria
Problems with histories
- "Donors may give inaccurate information intentionally or unintentionally, or because they misunderstand the question posed, or because they are unaware or have forgotten that they previously have had malaria."
Slinger et al (2001) Can Med Assoc J. 164: 377
Time Exclusion: UK Imported Malaria
Time to Presentation (2005)
| <1m | 1-5m | 6-11m | |
| Falciparum | 90% | 98.9% | 99.4% |
| Vivax | 42% | 75% | 95.7% |
| Ovale | 25% | 76% | 98.3% |
| Malariae | 36% | 100% |
Antibody Testing by IFAT
Draper and Sirr (1980)
- UK residents and immigrants with malaria
- One week after onset of symptoms
78% of UK residents
100% of immigrants
were seropositive
Draper and Sirr (1980)
Immigrant patients had
- Higher mean IFAT titres
- Longer persistence of antibodies
- Greater cross-reactions with other (non-falciparum) malarial antigens
Antibody Testing by ELISA
Chiodini et al (1997)
ELISA (P.falciparum antigen-based) in UK donor population:
- 0.45% seropositivity in non-TA donors
- 1.5% seropositivity in TA donors
- Could safely retrieve 40,000 red cell units discarded each year in Great Britain
- But in 1999 the assay was withdrawn from use in the Blood Service due to concerns over its performance
- In 2001 the IFAT (Voller & O'Neill 1971) was introduced in NTML at NBS North London
- Alternative assays were evaluated
Malaria Antibody EIA
(Newmarket Laboratories, UK)
- Sequential antibody sandwich EIA
- Recombinant protein antigens:
P.falciparum MSP1 and 2 MSP2
P.vivax MSP1 - Run in parallel to IFAT until May 2003
Malaria Antibody EIA and IFAT Testing
Serum samples tested:
- Acute malaria: within 7 days of 1st +ve film
- Follow-up: > or = 8 days after 1st +ve film
- Routine blood donations: no identified malaria risk
- Malaria-risk donations: malaria exposure history, returned 6 to 12 months ago
| ACUTE Species |
IFAT +ve |
NMK +ve |
Both +ve |
IFAT +ve NMK neg |
IFAT neg NMK +ve |
Both neg |
| P.falciparum (n=138) |
103 74.3% |
114 82.6% |
103 | 0 | 11 | 24 |
| P.vivax (n=13) |
8 61.5% |
11 84.6% |
8 | 0 | 3 | 2 |
| P.ovale (n=10) |
8 80% |
7 70% |
7 | 1 | 0 | 3 |
| FOLLOW-UP Species |
IFAT +ve |
NMK +ve |
Both +ve |
IFAT +ve NMK neg |
IFAT neg NMK +ve |
Both neg |
| P.falciparum (n=46) |
46
100% |
44 95.7% |
44 | 2 (Days 9 & 31) |
0 | 0 |
| P.vivax (n=5) |
3 60% |
4 80% |
3 | 0 | 1 | 1 |
| P.ovale (n=4) |
3 75% |
2 50% |
2 | 1 | 0 | 1 |
| DONOR CATEGORY |
IFAT +ve |
NMK +ve |
Both +ve |
IFAT +ve NMK neg |
IFAT
neg NMK +ve |
Both neg |
| Low risk (n=880) |
NT | 0 | N/A | N/A | N/A | N/A |
| Malaria risk (n=13,053) |
550 4.21% |
714 5.47% |
262 | 288 | 452 | 12051 |
Learning Effect with IFAT
| Date | Sera | IFAT | EIA | Both |
| (n) | only + | only + | +ve | |
| Ja 02 | 792 | 8.96% | 4.54% | 2.52% |
| M 02 | 576 | 3.12% | 3.3% | 3.82% |
| A 03 | 917 | 1.09% | 3.48% | 1.31% |
| M 03 | 990 | 0.61%; | 2.92% | 1.51% |
EIA Negative, IFAT Positive Donor Sera
January to April 2003
| IFAT Titre | No of sera |
|---|---|
| 1 in 10 | 1 |
| 1 in 20 | 7 |
| 1 in 30 | 13 |
| 1 in 40 | 7 |
| 1 in 60 | 3 |
| TOTAL 31 |
Additional data for non-falciparum malarias
- The following 2 slides show data for the Newmarket ELISA on acute and follow-up sera from patients with P.malariae, P.vivax and P.ovale infections. This information consists of results from the 2004 Vox Sanguinis paper (shown in the preceding slides in this presentation) and from samples tested since the 2004 publication combined into one data set.
| ACUTE Combined |
IFAT +ve |
NMK +ve |
Both +ve |
IFAT +ve NMK neg |
IFAT neg NMK +ve |
Both neg |
| P.malariae (n=13) |
12 92% |
10 77% |
10 | 2 | 0 | 1 |
| P.vivax (n=47) |
32 68% |
40 85% |
32 | 0 | 11 | 4 |
| P.ovale (n=36) |
32 89% |
26 72% |
26 | 6 | 0 | 4 |
| FOLLOW-UP Combined |
IFAT +ve |
NMK +ve |
Both +ve |
IFAT +ve NMK neg |
IFAT neg NMK +ve |
Both neg |
| P.malariae (n=1) |
1 | 1 | 1 | 0 | 0 | 0 |
| P.vivax (n=8) |
5 63% |
7 88% |
5 | 0 | 2 | 1 |
| P.ovale (n=5) |
3 60% |
2 40% |
2 | 1 | 0 | 2 |
NBS Donor testing for antimalarial antibodies
| Year | No. tested | RR | % |
|---|---|---|---|
| 2004 | 42947 | 1209 | 2.82 |
| 2005 | 66994 | 1368 | 2.04 |
| J-M 06 | 11988 | 236 | 1.97 |
NBS Donor testing in England, March 2006
HBsAg 19140 [new donors]
Malaria 4258
Universal testing for malaria antibodies would add another 14882 tests
UK Donor Selection Guidelines
Implemented November 2005
OBLIGATORY
Must not donate if:
- The donor has ever had malaria
- The donor has had an undiagnosed fever (which could have been malaria) while abroad or within six months of leaving a malaria endemic area
- The donor has lived in any malarial endemic area for a continuous period of six months or more at any time of life
- Less than 12 months after last leaving a malaria endemic area
DISCRETIONARY
Donors who have had malaria diagnosed:
- If more than three years have passed since anti-malarial therapy has been completed and symptoms caused by malaria have resolved, perform a validated test for malaria antibody. If this is negative, accept
DISCRETIONARY
For other donors:
- If at least six months has passed since the date of the last potential exposure to malaria, or the date of recovery from symptoms that may have been caused by malaria, a validated test for malaria antibody is negative, accept.
Conclusions
- Antibody testing provides a safeguard which is both additional and complementary to history taking and time exclusion and should not be seen as a replacement for those measures in the prevention of transfusion-transmitted malaria
- The UK policy of selective antimalarial antibody screening of donors with possible malaria exposure facilitates earlier donor reinstatement, without detracting from the current safety of the blood supply
Acknowledgements
- UK National Blood Service
Ms Patricia Lowe; Dr Alan Kitchen;
Prof John Barbara - LSHTM
Prof Chris Whitty; Prof Eleanor Riley - Hospital for Tropical Diseases
Mr Kalim Lalloo; Mr John Bligh


