 |
|
 |
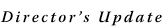

Pediatric Oncology Partnerships Are Models for Success
In this special issue of the Bulletin, we pay tribute to pediatric oncology, focusing on the wide-reaching partnerships that have led to exemplary progress in treating children with cancer. As you will see, these partnerships have markedly reduced pediatric cancer mortality and will most certainly hasten our progress against other cancers, such as osteosarcoma 1 and brain tumors 2, for which prognosis remains poor.
NCI sponsors several cooperative clinical trials groups to study pediatric cancers, the largest of which is the Children's Oncology Group (COG 3). In 2000, NCI facilitated the formation of COG through a merger of the Children's Cancer Group, the Pediatric Oncology Group, the Intergroup Rhabdomyosarcoma Study Group, and the National Wilms Tumor Study Group. Because of this network, accrual to pediatric clinical trials is remarkably high: Among eligible children younger than 5, 90 percent or more are currently treated as part of a clinical trial, compared with less than 5 percent participation in trials by the adult population.
NCI's Pediatric Oncology Branch 4 is a member of COG. Through the Advanced Technology Program 5 at NCI-Frederick and the Office of Science and Technology Partnerships 6, the Pediatric Oncology Branch has unique access to technology and procedures for genetic analysis, biomarker studies, and targeted therapy development - advances that can be tested in pre-clinical and early-phase clinical trials before moving to the larger extramural community.
Read more 7 

International Ewing Sarcoma Study Under Way
Researchers at NCI have joined forces with investigators across the U.S. and Europe to launch an international clinical trial of a promising new agent against Ewing sarcoma 8, a rare cancer that affects mostly children, adolescents, and young adults.
The agent, called R1507, is an investigational monoclonal antibody produced by Hoffmann-La Roche that inhibits insulin-like growth factor 1
receptor (IGF-1R). Ewing sarcoma has been linked with mutated genes that promote the production of IGF-1R. Previous phase I studies that included adolescents and young adults demonstrated promising results in Ewing sarcoma patients with treatment-resistant (refractory), progressive disease who had failed multiple standard and "salvage" therapies. In some cases, there have been complete responses to IGF-1R blockers in such high-risk patients.
Dr. Lee Helman, NCI's scientific director for clinical science and a noted expert in pediatric sarcomas, received numerous calls about these early results and met with his colleagues as part of the Sarcoma Alliance for Research through Collaboration several times to hear presentations from companies, including Roche, about their IGF-1R blocker compounds for use in a planned phase II 9 study in children and adults aged 12 years and older with relapsed or refractory Ewing sarcoma. They selected R1507 for the current study and will expand this treatment to several other pediatric and adult sarcomas, including rhabdomyosarcoma 10 and osteosarcoma 1.
Read more 11 
 |
The NCI Cancer Bulletin is produced by the National Cancer Institute (NCI). NCI, which was established in 1937, leads the national effort to eliminate the suffering and death due to cancer. Through basic, clinical, and population-based biomedical research and training, NCI conducts and supports research that will lead to a future in which we can identify the environmental and genetic causes of cancer, prevent cancer before it starts, identify cancers that do develop at the earliest stage, eliminate cancers through innovative treatment interventions, and biologically control those cancers that we cannot eliminate so they become manageable, chronic diseases.

For more information on cancer, call 1-800-4-CANCER or visit http://www.cancer.gov.

NCI Cancer Bulletin staff can be reached at ncicancerbulletin@mail.nih.gov.
|
|
 |
 |

Pediatric Oncology Partnerships Are Models for Success
In this special issue of the Bulletin, we pay tribute to pediatric oncology, focusing on the wide-reaching partnerships that have led to exemplary progress in treating children with cancer. As you will see, these partnerships have markedly reduced pediatric cancer mortality and will most certainly hasten our progress against other cancers, such as osteosarcoma 1 and brain tumors 2, for which prognosis remains poor.
NCI sponsors several cooperative clinical trials groups to study pediatric cancers, the largest of which is the Children's Oncology Group (COG 3). In 2000, NCI facilitated the formation of COG through a merger of the Children's Cancer Group, the Pediatric Oncology Group, the Intergroup Rhabdomyosarcoma Study Group, and the National Wilms Tumor Study Group. Because of this network, accrual to pediatric clinical trials is remarkably high: Among eligible children younger than 5, 90 percent or more are currently treated as part of a clinical trial, compared with less than 5 percent participation in trials by the adult population.
NCI's Pediatric Oncology Branch 4 is a member of COG. Through the Advanced Technology Program 5 at NCI-Frederick and the Office of Science and Technology Partnerships 6, the Pediatric Oncology Branch has unique access to technology and procedures for genetic analysis, biomarker studies, and targeted therapy development - advances that can be tested in pre-clinical and early-phase clinical trials before moving to the larger extramural community.
NCI's Pediatric Oncology Branch led partnerships that facilitated the successful completion of several therapeutic advances that were first tested in children at the NIH Clinical Center 12. Among them was the first use of gene therapy, development of "volume photography" to measure growth of neurofibromatosis-1 tumors, and even the first multi-institute hospital unit designed specifically for children at the NIH Clinical Center.
NCI's Pediatric Oncology Branch often works closely with other NIH institutes to cosponsor important pediatric research projects, combining valuable resources and thereby speeding the delivery of new interventions. And collaboration between NCI's SEER 13 Program, Office of Cancer Survivorship 14, and organizations such as the Lance Armstrong Foundation 15, help us monitor pediatric survivorship issues such as fertility, second cancers, and race and age-group disparities.
Children usually learn from adults, but sometimes the roles are reversed, and that has been true in pediatric oncology. Some fundamental aspects of cancer treatment today, such as combination chemotherapy and knowledge of tumor suppressor genes, can be traced to research first completed on pediatric cancer patients. Such advances appear in our "Milestones 16."
You may also notice the banner that spans the top of this special issue. It features artwork made by children who have stayed at the Children's Inn at NIH 17 while undergoing cancer treatment, or children who attended therapy sessions led by Dr. Lori Wiener, a social worker and member of our Pediatric Oncology Branch. Both of these programs are highlighted in this special issue.
We at NCI are extremely proud of our Pediatric Oncology Branch and the rich interface of intramural and extramural research in childhood cancers. We always get a special lump in our throats and a smile when we have a successful outcome with one of our special kids.
Dr. John E. Niederhuber
Director, National Cancer Institute
|
 |
 |

Researchers at NCI have joined forces with investigators across the U.S. and Europe to launch an international clinical trial of a promising new agent against Ewing sarcoma 8, a rare cancer that affects mostly children, adolescents, and young adults.
The agent, called R1507, is an investigational monoclonal antibody produced by Hoffmann-La Roche that inhibits insulin-like growth factor 1
receptor (IGF-1R). Ewing sarcoma has been linked with mutated genes that promote the production of IGF-1R. Previous phase I studies that included adolescents and young adults demonstrated promising results in Ewing sarcoma patients with treatment-resistant (refractory), progressive disease who had failed multiple standard and "salvage" therapies. In some cases, there have been complete responses to IGF-1R blockers in such high-risk patients.
Dr. Lee Helman, NCI's scientific director for clinical science and a noted expert in pediatric sarcomas, received numerous calls about these early results and met with his colleagues as part of the Sarcoma Alliance for Research through Collaboration several times to hear presentations from companies, including Roche, about their IGF-1R blocker compounds for use in a planned phase II 9 study in children and adults aged 12 years and older with relapsed or refractory Ewing sarcoma. They selected R1507 for the current study and will expand this treatment to several other pediatric and adult sarcomas, including rhabdomyosarcoma 10 and osteosarcoma 1.
"Because these are rare tumors, no single institution can do a large enough study - nor can any one country," Dr. Helman commented, noting that there are only about 200 new cases of Ewing sarcoma diagnosed in the United States each year. "We very quickly engaged our European colleagues who we've collaborated with on a number of previous projects."
The trial got under way at several U.S. cancer centers in December and has already accrued about 30 patients. The study will open shortly at the NIH Clinical Center and at sites in France, Italy, Germany, and the United Kingdom next month.
Dr. Herbert Juergens, professor of pediatric hematology and oncology at the University of Muenster, which is one of the study sites in Germany, said that progress in treatment of Ewing sarcoma "has become very slow" in recent years. Research has achieved long-term survival rates of about 70 percent with standard treatments, which include intensive chemotherapy and "local" interventions of radiation and surgery. "Since then, we seem to have exhausted the intensity of chemotherapy we can administer to the patients," he continued. Instead, hope has focused on finding therapies that target specific receptors in these tumors.
The international trial to test R1507 for Ewing sarcoma "is wonderful," Dr. Juergens said. To find sufficient patients with relapsing disease to produce statistically significant data, he added, "You need to work across the Atlantic Ocean."
Dr. Helman noted, "Over the next several years, we'll have to work hard to see if we can identify genomic profiles or markers that will predict those patients who are more likely to respond to IGF-1R inhibitors and figure out how we might move this to frontline therapy, because it might minimize toxicity" compared with current chemotherapy.
|
 |
 |

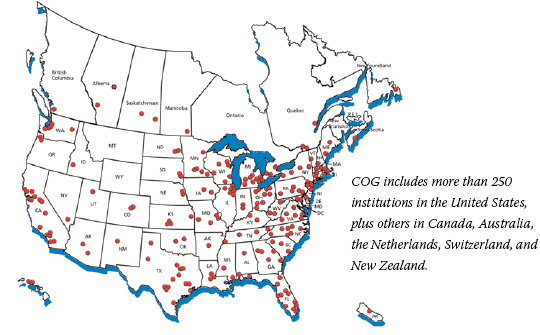
Fifty years ago, less than 10 percent of children diagnosed with cancer survived long term. Today, that number is almost 80 percent overall. This radical improvement comes largely from the efforts of the Children's Oncology Group (COG 3), the nation's largest cooperative clinical research group.
The first U.S. pediatric clinical trials group formed in 1955 and received NCI funding in 1956 to address the difficulty of performing clinical trials for children's cancers. In 2000, this group and other U.S. pediatric trial groups merged to form the COG.
"It was recognized that no individual cancer program or children's hospital had the number of patients that could support clinical investigation by themselves," says Dr. Gregory Reaman, professor of pediatrics at The George Washington University and chair of COG. "Cooperation and collaboration were absolutely necessary."
 |
 |
Pediatric clinical trial
enrollment by eligibility

5 and younger: greater than 90%
10 and younger: 75-90%
10 to 15: 50%
Adolescents aged 15 to 19: 15-25%
|
 |
|
|
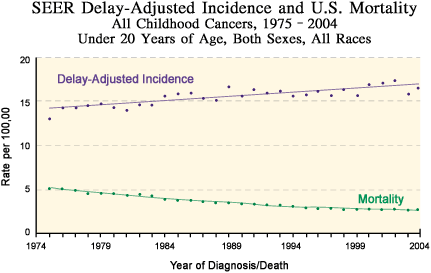
The first cooperative clinical trials focused on chemotherapy for acute lymphoblastic leukemia (ALL 18), the most common childhood cancer. Rapid improvements for ALL through this system led the groups to expand their research to other hematologic cancers, as well as solid tumors, adding surgery, radiation therapy, immunotherapy, and newer targeted treatments to their experimental regimens.
Today more than 40,000 children are treated on or receive followup as part of COG clinical trials at hundreds of hospitals around the world. In the U.S., "the majority of children for whom there are clinical trials, and who are eligible for those trials, are actually enrolled on clinical trials," says Dr. Reaman. This stands in stark contrast to adults, where only a few percent of eligible patients participate in clinical research.
One of COG's main goals is to increase the number of children treated on clinical trials, but they face many of the same barriers to enrollment encountered by researchers working with adult patients, including scarce funding, lack of access for patients in rural areas, and difficulty in some cases getting insurance companies to cover experimental care.
 |
 |
CureSearch - the foundation affiliated with the COG - recently launched a Spanish-language version of its award-winning pediatric cancer Web site at www.curesearch.org/spanish
|
 |
|
|
In addition, explains Dr. Maura O'Leary, administrative officer with COG, for patients who speak English as a second language, the process of informed consent can be complicated. "The ideal would be to print [consent forms] in their own language," she says, so COG has recently started a pilot program to create Spanish-language materials.
In addition to examining the genetics of childhood cancers, COG plans to expand their cancer control, prevention, supportive care, and survivorship research, with international partnerships. "Certain cancers that are rare in the U.S. are more common in other parts of the world, such as Latin America and India," says Dr. O'Leary. "If we can work together to learn how to better treat those cancers, everybody will benefit."
|
 |
 |

Because of the rarity of cancer in children and adolescents, for researchers investigating childhood cancer risks, it's proven difficult to get large enough study populations that can provide reliable, statistically valid conclusions about factors that may only moderately increase cancer risk.
However, through increased collaboration, researchers are retooling their approaches in hopes of getting stronger data on such factors. Their goal is to answer some difficult questions about how a disease like cancer, which is thought to take years or even decades to develop, can take hold in humans within just a few years - or sometimes months - of birth.
"If it's a huge risk, you don't necessarily need enormous numbers to be able to identify the risk factor," explains Dr. Martha S. Linet, chief of the Radiation Epidemiology Branch in NCI's Division of Cancer Epidemiology and Genetics (DCEG 19). Down syndrome, for example, increases childhood leukemia 20 risk 40-fold. But most childhood leukemia case-control studies, Dr. Linet continues, have identified factors with far smaller risks.
"When these small risks are discovered, it's very difficult to tease apart if they're real, or due to some confounding factors," Dr. Linet says.
The International Childhood Cancer Cohort Consortium 21 is designed to address this problem. Comprising 11 cohorts - none of which was established to study cancer - this international consortium involves more than 70,000 children who are enrolled by their parents before or at birth and, depending on which cohort, will be followed for varying time periods. Each cohort study, while not identically designed, includes detailed questionnaires about potential exposures and the collection of biological samples for molecular analyses.
"Over time," Dr. Linet says, "we're going to be exploring some promising hypotheses that are difficult to study in case-control studies."
Exploration of genetic risks for pediatric cancer has also been limited by small numbers. Such research has mostly been relegated to familial cancers or cancers associated with syndromes such as Li-Fraumeni.
"We've learned a lot from the cancer predisposition syndromes," says Dr. Sharon Savage, an investigator in DCEG's Clinical Genetics Branch. But, because of the rarity of childhood cancers, "We don't yet have a good understanding of how common genetic variants like SNPs (single nucleotide polymorphisms) may affect risk."
Despite the small sample sizes, some headway is being made. In an August 2007 Cancer Epidemiology, Biomarkers & Prevention 22 study, for instance, Dr. Savage and NCI colleagues analyzed blood samples from 104 patients with osteosarcoma 1, which is most prevalent in adolescent boys, and found a two-fold increased risk associated with SNPs in IGF2R, a critical gene for growth regulation.
But now they need more numbers. And, with the help of the Children's Oncology Group (COG), they will try to replicate the study with a larger sample size, using 500 to 600 tumor samples from osteosarcoma patients enrolled in COG trials.
Although it's still unclear what this line of investigation will ultimately mean for the prevention or treatment of osteosarcoma, Dr. Savage says, "We're hopeful that it can inform osteosarcoma research as well as other research into cancers in which similar pathways play a role."
|
 |
 |

 |
 |
Children's Inn at NIH



The Children's Inn at NIH provides lodging, healing services, and programs for seriously ill children and their families who are treated across the street at the NIH Clinical Center. To learn more, go to http://www.childrensinn.org.
|
 |
|
|
Childhood cancers are biologically different from those that arise later in life. Cancers in children are more likely to involve developing organs, for instance, or to begin in the prenatal environment.
But whether a cancer occurs early or late in life, the path to more effective and less toxic therapies is essentially the same - it starts with knowing the genetic changes underlying the disease. The tools for discovering these changes may also be the same in childhood and adult cancers, as an innovative public-private initiative hopes to demonstrate.
The initiative, sponsored by NCI and the Foundation for the National Institutes of Health, is called TARGET 23 (the Childhood Cancer Therapeutically Applicable Research to Generate Effective Treatments). The goal is to identify and validate therapeutic targets for childhood cancers using the latest genetic and genomic tools, which are the same ones used to develop targeted drugs for adult cancers.
"This project was inspired by the powerful technologies that are available for identifying genetic lesions in cancer cells," said Dr. Malcolm Smith of NCI's Cancer Therapy Evaluation Program 24 and a leader of TARGET. The initiative grew out of a 2005 workshop among cancer specialists from the public and private sectors. A consensus emerged that available technologies could advance research on childhood cancer therapies if sufficient resources were dedicated to the challenge.
A pilot phase of TARGET involving acute lymphoblastic leukemia (ALL 18) and led by researchers from the University of New Mexico, St. Jude Children's Research Hospital, the Children's Oncology Group, and NCI has produced interesting early results. Profiling gene activity in patient samples revealed a previously unknown subtype of ALL associated with a poor prognosis. Additional studies are underway to search for genomic abnormalities such as extra copies of genes that may underlie the abnormal gene activity detected in the patient samples.
With the diversity of data, the researchers expect eventually to identify the underlying genetic changes in this high-risk subgroup of children with ALL. "The challenge is to identify the genetic lesions that in one way or another are driving the leukemia," noted Dr. Smith.
The initiative is benefiting from lessons learned and tools developed during the implementation of The Cancer Genome Atlas 29 pilot project, which is cataloging genomic alterations in three adult cancers. Both projects will share, for instance, software designed to make the results easily accessible to researchers via the Internet.
The integration of different types of data will be an ongoing process in TARGET. But the researchers believe that by studying relatively large numbers of patients, using consistent research methods, and sequencing between 100 and 150 genes per disease, they will be able to identify critical genes in these cancers that can lead to more effective treatments.
The pilot project is also working on neuroblastoma 30, with plans to expand to rhabdomyosarcoma 10 and other cancers in the future.
|
 |
 |

 It is perhaps a good problem to have: Many more experimental cancer drugs enter clinical evaluation in adults each year than can realistically be tested in children, given the small number of children with cancer eligible for early-stage clinical trials.
It is perhaps a good problem to have: Many more experimental cancer drugs enter clinical evaluation in adults each year than can realistically be tested in children, given the small number of children with cancer eligible for early-stage clinical trials.
This means that investigators have to prioritize agents, and their decisions have consequences. If 20 new drugs are available for study, but only one or two can be tested in children each year, then picking an agent that lacks an effect against childhood cancers may delay or prevent an agent that is truly effective from being investigated.
So how can investigators improve their chance of making good decisions about which new agents to bring forward for pediatric clinical testing? One way may be to test candidate agents in the laboratory using pediatric cancer cell lines and animal models. Launched in 2004, the NCI-sponsored Pediatric Preclinical Testing Program (PPTP) 31 is doing this by testing 12 new agents a year against its panel of childhood cancer preclinical models.
The project is an experiment, and it will not be known for several more years how well the models predict anticancer activity in patients. But early results are encouraging. The researchers have provided validation for the strategy by showing that standard chemotherapy drugs have similar effects in the preclinical models as in patients. Importantly, the program has already identified several novel agents that show substantial activity against one or more of the PPTP's childhood cancer tumor panels.
A recent PPTP report in Pediatric Blood Cancer describes a drug with anticancer activity against several solid tumors. The drug, SCH 717454 32, is a monoclonal antibody against the insulin-like growth factor 1 receptor (IGF-1R), which has been implicated in many pediatric solid tumors. Another PPTP publication reported that ABT-263 33, a small molecule inhibitor of Bcl-2 family proteins, has substantial single agent activity against acute lymphoblastic leukemia 18 cell lines and xenografts.
"The project is providing useful ideas about new agents and combinations of therapies that can be studied in children," said principal investigator Dr. Peter Houghton of St. Jude Children's Research Hospital. The PPTP's research teams have more than 60 animal models and 27 cell lines available for testing, representing most of the more common cancers that occur in children.
The program tries to evaluate new agents near the time that they are entering clinical trials in adults. This allows pediatric preclinical data to be developed while adult phase 1 trials are ongoing so that the preclinical data can then inform decision-making about clinical evaluations of agents tested in children. The adult trials provide information about the drug blood levels that are tolerated in humans, and this information can be modeled in mice to learn whether children are likely to tolerate a dose of the agent that is active against preclinical models.
"The critical issue in terms of translation from the preclinical model to the clinic is using doses in the animal that produce blood levels that you can achieve in the patient," noted Dr. Houghton.
More than 20 pharmaceutical companies have submitted one or more agents for testing. "The willingness of these companies to collaborate with the PPTP has been very encouraging and is central to the success of the program," said Dr. Malcolm Smith of NCI's Cancer Therapy Evaluation Program, who oversees the PPTP.
|
 |
 |

Acting Chief of the Pediatric Oncology Branch (POB) in NCI's Center for Cancer Research (CCR 34)
 How does the POB augment work in the extramural community? How does the POB augment work in the extramural community?
Progress against cancer in children has benefited greatly from well-organized cooperative groups in the extramural community that conduct efficient and large randomized trials. However, future progress hinges upon biologic insight and targeted therapies, both of which begin in a basic science laboratory. The focused basic and clinical science conducted in the POB provides the basis and rationale for new therapies, which, if successful, would ultimately be tested in the extramural community.
In what case would a child, adolescent, or young adult come to clinicians in the POB at the NIH Clinical Center in Bethesda for treatment?
The POB conducts clinical trials targeting a variety of childhood cancers and cancer predisposition syndromes for which outcomes with standard therapies are suboptimal. Any child, adolescent, or young adult who is eligible for such trials may be treated in the POB. In some cases, this represents frontline therapy for a newly diagnosed cancer and in others, the therapies are tested in the setting of recurrent disease. A list of POB protocols can be found at http://pediatrics.cancer.gov/protocol.shtml.
Why is pediatric oncology appropriate for patients who would under other circumstances be considered adults?
Cancers that are most common in children but also afflict adults are often treated by pediatric oncologists familiar with the biology and behavior of those diseases. For example, Ewing sarcoma 35 usually occurs in the second decade of life, but may also occur during early and mid-adulthood and, from a molecular standpoint, is the same disease regardless of the age at which it strikes. Treatment regimens used for adults with this disease are the same as those used for children and therefore many current studies for Ewing sarcoma allow both children and adults to be treated on the same trial.
What are examples of cutting-edge research this year in the POB?
Historically, the Molecular Oncology Section of the POB, under the direction of Dr. Lee Helman, contributed seminal work defining the critical role for IGF-1R signaling in pediatric sarcomas. Monoclonal antibodies targeting this receptor have recently entered phase I clinical trials with encouraging results. There is great optimism in the pediatric sarcoma community that this may represent the first biologically based targeted therapy to show activity in these diseases. In a second example, the Hematologic Diseases Section, under the direction of Dr. Alan Wayne, is working closely with investigators from the Laboratory of Molecular Biology at NCI to develop anti-CD22-based immunotoxins to target acute lymphoblastic leukemia (ALL 18). A recent phase I study 36 showed clinical activity using this approach in patients with recurrent ALL and plans are underway to improve it with a newer, higher-affinity version of the toxin conjugate and combination with standard chemotherapy, which was synergistic in preclinical models. Recurrent ALL remains the single greatest killer of children with cancer and we are very hopeful that this new biologically based therapy will provide new options for patients.
To learn more about the Pediatric Oncology Branch please visit: http://home.ccr.cancer.gov/oncology/pediatric/.
|
 |
 |
 |

Compared with advances achieved for younger children over the past 30 years, there has been a relative lack of progress in identifying more effective treatments for adolescent and young adult (AYA 37) cancer patients. Cancer survival rates for AYA patients, who are those diagnosed with cancer at ages 15 through 39, have seen little or no improvement for decades. This concern led NCI and the Lance Armstrong Foundation (LAF 15) in 2005-2006 to convene a Progress Review Group (PRG) to evaluate the issues behind this bleak trend.
 |
 |
The 2008 NCI SEER Cancer Statistics Review includes a new chapter on AYA cancers and will be posted online in May at http://seer.cancer.gov/
|
 |
|
|
In its final report 38, the PRG described factors that contribute to the problem: high numbers of uninsured AYAs and a tendency for kids and young adults with cancer to fall into a "no man's land" between pediatric and adult oncology practices. The panel concluded that "a major, ongoing AYA [oncology]-specific research initiative emphasizing AYA clinical trials and outcomes research is urgently needed."
Now a trans-NCI study of AYA patients, led by the Division of Cancer Control and Population Sciences 39, is under way in seven SEER registries with support from the LAF. The study will investigate patterns of care and outcomes for patients aged 15-39 with acute lymphoblastic leukemia (ALL 18), lymphoma 40, sarcoma 41, and germ cell cancers through a patient survey and medical record examination. The study will assess treatment, physician and facility characteristics, clinical trials participation, barriers to care, as well as the impact of cancer on physical symptoms, psychosocial experiences, financial issues, and quality of life.
Dr. Malcolm Smith of NCI's Cancer Therapy Evaluation Program also points to clinical trials for AYA patients with ALL. "Recent publications have reported that adolescents and young adults treated on pediatric clinical trials have had better outcomes than similarly aged AYA patients treated using adult protocols for ALL," he noted.
One of the trials cited by Dr. Smith is being conducted by the Dana-Farber Cancer Institute (DFCI) Consortium, which presented preliminary findings 42 at the American Society of Hematology annual meeting last year. Current chemotherapy regimens in children with ALL produce event-free survival (EFS) rates of greater than 80 percent compared with EFS rates of 30-40 percent in adults with ALL. DFCI Consortium researchers reported that for patients aged 18-50 with ALL who were treated with an intensive pediatric regimen, the estimated 2-year EFS was 72.5 percent. Although the study requires longer follow up, the preliminary results suggest that intensive treatment strategies for young adults with ALL could represent a major therapeutic advance.
Another study to improve outcomes for young adults with ALL, CALGB-10403 43, is under way with the NCI-sponsored adult cooperative groups. The study adopts the treatment approach for ALL that is used by the Children's Oncology Group (COG). A retrospective analysis conducted as part of the trial compared outcomes of ALL patients aged 16-20 who were treated by either the CALGB Intergroup physicians or by pediatric COG specialists.
"What we found was that there was tremendous disparity in outcomes and that those AYA patients who were treated on pediatric regimens had significantly improved disease-free survival and overall survival," said Dr. Wendy Stock, associate professor at the University of Chicago Department of Medicine. "It was almost a 30 percentage point difference. That was very disappointing and it stimulated a lot of questions about why this might be."
The Intergroup retrospective analysis, which will be published in Blood, found key differences in both the dose intensity of the drugs used and in the treatment schedules adopted by pediatric versus adult cancer specialists. "In addition to differences in the protocol design and schedule, the outcome disparity also raises questions about protocol compliance and whether adult medical oncologists and the patients that they treat adhere to protocols as rigorously as the pediatric oncologists and their patients," Dr. Stock noted. "We hope to begin to gather specific data about treatment compliance in addition to exploring the feasibility of using this approach to improve outcomes of AYAs with ALL in the adult cooperative groups."
|
 |
 |

In addition to watching a loved one undergo difficult treatments, families of children with cancer must deal with disruption of normal life, travel to treatment centers, arranging childcare, finances, and other challenges. The cumulative effects can have serious consequences for quality of life during treatment and after it ends.
"We realize now that in order to provide our pediatric patients with the services that they need, we have to see these children in the context of their families and their communities, and integrate each of these dimensions so they can return home with the support, tools, and coping strategies to survive cancer," explains Dr. Lori Wiener, head of the Psychosocial Section and coordinator of the Psychosocial Support and Research Program in NCI's Pediatric Oncology Branch 4.
She hopes that some of the research under way through her program, which has developed resources and practice models for treatment centers and families, can help people who are looking for ways to handle these challenges. Psychosocial support within the pediatric oncology program begins with social workers who contact patients as soon as they are recommended for a clinical trial at NIH, and who maintain contact with these families one-on-one throughout the course of their treatment.
 Many of these families stay at the Children's Inn at NIH, where support programs compliment services at the Clinical Center, including play, art, and music therapy; support groups and workshops; psychiatric consultation; counseling; camp programs; school programs and vocational testing; pain and palliative care services; spiritual ministry; and bereavement programs, among others.
Many of these families stay at the Children's Inn at NIH, where support programs compliment services at the Clinical Center, including play, art, and music therapy; support groups and workshops; psychiatric consultation; counseling; camp programs; school programs and vocational testing; pain and palliative care services; spiritual ministry; and bereavement programs, among others.
Psychotherapy for children in clinical trials also addresses fear and anxiety due to being far from home, new treatments, and invasive procedures. "It's horrifying to see a panic-stricken child trying to deal with an impending bone marrow transplant, or any kind of needle stick," says Dr. Jimmie Holland, a psychiatrist and Wayne Chapman Chair in Psychiatric Oncology at Memorial Sloan-Kettering Cancer Center, which has the country's largest training program in this field. "The experience upsets anyone who comes near it. But if you can help these children and their family members prepare for the rigors of treatment, using behavioral techniques that are proven, you can give patients and their families a sense of control and diminish panic in these situations."
Drs. Holland and Wiener have worked together on resources that outline psychosocial issues and solutions that are so critical to successful cancer care, some of which are based on a report commissioned by the Institute of Medicine, Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs 44, as well as a reference book that will soon be available from the American Psychosocial Oncology Society, Quick Reference for Pediatric Oncology Clinicians: The Psychiatric and Psychological Dimensions of Pediatric Cancer Symptom Management 45, for which Dr. Wiener is lead editor.
Dr. Wiener's program has also developed tools, including several for the siblings of cancer patients: a workbook titled This is MY World, another titled Brothers and Sisters - We're in This Together, and a customizable board game available later this year.
After children complete their cancer treatment and leave the Clinical Center, they are connected with the support network in their local communities. But their relationship with staff at NIH can last a lifetime. Even when a child dies, their family members are considered cancer survivors, Dr. Wiener explains, and she is in touch with them each year on the anniversary of their loss. "There's a sense that we've lived through this life-altering experience together," Dr. Wiener says, "and a special connection often arises between families and the health care providers who traveled that journey with them."
|
 |
 |

The very high cure rate in pediatric oncology has not been achieved without a cost: treatments that save children's lives can also damage developing organs, causing a wide array of health problems that may not surface for many years.
Current understanding of the extent of "late effects" in childhood cancer survivors - health problems related to cancer treatment that occur months to years after treatment ends - has emerged largely from the Childhood Cancer Survivors Study (CCSS 46). Since 1994, this NCI-funded research project has followed a cohort of 20,000 people who were diagnosed with cancer as children between 1970 and 1986 and survived at least 5 years.
In a study published in the New England Journal of Medicine 47 in 2006, which looked at outcomes in more than 10,000 survivors, CCSS researchers found that almost two-thirds of patients reported at least one chronic health problem, one-quarter had a severe condition, and almost one-quarter had three or more chronic health problems. Late effects reported most frequently in this study were second cancers, cardiovascular disease, kidney disease, musculoskeletal conditions, and endocrine abnormalities. The risk of developing a health problem related to cancer treatment in childhood increased over time.
Women face higher risks than men for late effects including breast cancer, cognitive dysfunction, heart disease, and hypothyroidism. Other factors influencing late effects include age at diagnosis, type of cancer, and types of treatment received. Radiation treatment, especially to the brain - and, in women, the chest - carries a high risk of long-term effects.
"Both the magnitude and the diversity of the long-term health effects have been striking," says CCSS principal investigator Dr. Les Robison of St. Jude Children's Research Hospital in Memphis. "At 30 years after their diagnosis, more than 70 percent of childhood cancer survivors have a late-effect chronic health condition."
 Results from CCSS studies are now informing the development of guidelines for appropriate follow-up care for childhood cancer survivors, adds Dr. Robison. The limited data so far available suggest that most survivors do not receive the recommended follow-up care. CCSS researchers are now planning several new studies looking at how to ensure more survivors obtain follow-up care so that late effects are detected as early as possible.
Results from CCSS studies are now informing the development of guidelines for appropriate follow-up care for childhood cancer survivors, adds Dr. Robison. The limited data so far available suggest that most survivors do not receive the recommended follow-up care. CCSS researchers are now planning several new studies looking at how to ensure more survivors obtain follow-up care so that late effects are detected as early as possible.
CCSS data are available to researchers studying important questions in pediatric cancer survivorship through the St. Jude Web site (www.stjude.org/ccss). To date, the CCSS has generated more than 75 publications in the peer-reviewed literature.
Treatments for childhood cancer have changed a lot since the CCSS cohort was treated in the 1970s and early 1980s. To learn about the long-term effects of these newer therapies, researchers are now expanding the CCSS cohort by recruiting 20,000 survivors treated for childhood cancer between 1987 and 1999 who have survived at least 5 years.
In other efforts, researchers are developing new tools, such as the Passport for Care 48, to help clinicians and childhood cancer survivors be more aware of and communicate with each other about the long-term effects of cancer treatments, as well as decision aids 49 to help survivors weigh the costs and benefits of treatment approaches.
"Childhood cancer survivors are in the vanguard in terms of our understanding of the impact of living long-term after cancer treatment," says Dr. Julia Rowland, director of NCI's Office of Cancer Survivorship 50. "The challenge is to ensure that they are appropriately followed across their lifetimes to minimize cancer's late adverse impacts and maximize these survivors' overall health."
|
 |
 |

| General Information about Childhood Cancer |
| Reports on Childhood Cancer Incidence from NCI |
| Clinical Trials and Treatment Centers |
|
 |
 |

FDA Advisory Committee Recommends Further Limits on
Use of ESAs
On March 13, the U.S. Food and Drug Administration's (FDA) Oncologic Drugs Advisory Committee (ODAC) recommended substantially limiting the use of erythropoiesis-stimulating agents (ESAs) to treat anemia in cancer patients. The panel made the recommendation after hearing additional evidence from a recently published meta-analysis 51 showing that ESAs increase the risk of blood clots and death in patients taking the drugs for chemotherapy-induced anemia.
The committee recommended that the drugs be restricted to cancer patients with advanced disease; that the drugs not be used in patients with breast or head and neck cancer, the cancer types with the most evidence of severe side effects; and that patients be required to complete a written consent before using ESAs.
This was the third ODAC meeting 52 held to discuss safety concerns about ESAs. The
FDA isn't required to adopt the advisors' recommendations, but agency representatives indicated during the ODAC meeting that they are likely to adopt further restrictions on the use of ESAs. There are currently three FDA-approved ESAs on the U.S. market: Aranesp (darbepoetin alfa), Epogen (epoetin alfa), and Procrit (epoetin alfa).
|
 |
 |
 |
 |
Also in the News

Researchers funded by the National Institute of Allergy and Infectious Diseases report 53 that pre-existing immunoglobulin E antibodies are the cause of severe adverse reactions experienced by some patients given cetuximab 54 - an immune-based therapy commonly used to treat cancers of the head and neck and colorectal cancers. The findings are reported in the March 13 New England Journal of Medicine 55.
|
 |
|
|
Nonprotruding Colorectal Growths May Harbor Cancer
Most colorectal cancers are thought to arise from polypoid adenomas - growths that protrude from the mucous membrane in the colon or rectum. A study from the Veterans Affairs (VA) Health Care System in Palo Alto, CA, published in the March 5 Journal of the American Medical Association 56 adds to a growing body of evidence that nonpolypoid colorectal neoplasms (NP-CRNs) - abnormalities that can appear either flat or depressed relative to the surrounding membrane - can also contain precancerous or cancerous cells. Previous studies established the existence of NP-CRNs in Japan, but their prevalence and importance in other parts of the world has remained unclear.
In the VA study, gastroenterologists and pathologists who were trained in a special exchange program with Japanese cancer centers examined 1,819 patients at the Palo Alto VA hospital undergoing colonoscopy for screening, surveillance (for people at high risk of colorectal cancer), or symptoms of colorectal neoplasms. Patients underwent biopsy, removal of polyps or NP-CRNs, or surgery as needed.
The investigators found NP-CRNs in 170 patients (9.35 percent of those examined). Although these lesions were less common than colorectal polyps, they were more likely to contain precancerous or cancerous cells, accounting for only about 15 percent of identified neoplasms but 54 percent of superficial carcinomas. "Nonpolypoid morphology was strongly associated with findings of in situ or submucosal invasive carcinoma," stated the authors.
NP-CRNs missed during colonoscopy may help explain the occurrence of interval colorectal cancers - cancers that arise between scheduled screening colonoscopies in patients who appear not to have any precancerous polyps - explains Dr. David Lieberman from Oregon Health and Science University in an accompanying editorial 57.
The existence of NP-CRNs will have important implications for colorectal cancer screening in the United States, continued Dr. Lieberman. "The finding that NP-CRNs have high rates of serious pathology suggests that effective screening programs will need to accurately identify patients who harbor these lesions."
Delayed Letrozole Therapy After Tamoxifen Reduces Breast Cancer Recurrence
An analysis of data from a phase III clinical trial that was unblinded 5 years ago indicates that in some breast cancer patients, use of the aromatase inhibitor letrozole 58 (Femara) after 5 years of adjuvant therapy with tamoxifen 59 has survival benefits even if begun several years after completing tamoxifen.
Conducted by the National Cancer Institute of Canada Clinical Trials Group, the trial, known as MA.17, was unblinded in 2003 after an interim analysis demonstrated that letrozole, begun within 3 months after 5 years of tamoxifen, reduced breast cancer recurrence risk 60 compared to placebo. Participants in the placebo arm of MA.17 were then offered the opportunity to begin 5 years of adjuvant treatment with letrozole. MA.17 participants were post-menopausal women with early-stage, ER-positive disease.
This new analysis from MA.17, released online on March 10 by the Journal of Clinical Oncology (JCO) 61, compared the 1,579 women in the placebo arm who began using letrozole after the trial was unblinded with the 804 women who chose not to. The analysis revealed that letrozole improved disease-free survival by 63 percent and distant (or metastatic) disease-free survival by 61 percent. The median time of letrozole initiation after tamoxifen completion was 2.8 years, with a range from 1 to 7 years.
Questions about the optimal approach to adjuvant therapy in this patient population linger, the study's lead author, Dr. Paul Goss from Harvard Medical School, and colleagues noted, because even after 5 years of adjuvant tamoxifen, there continues to be a significant risk of late recurrence and death from breast cancer.
Although interpretation of the findings is "made difficult because this therapeutic intervention was self-selected, not randomly allocated," they continued, the women who took letrozole had disease characteristics that put them at higher recurrence risk than those who did not, suggesting letrozole was responsible for the beneficial effects.
Letrozole use was also associated with an increased risk of bone fractures and osteoporosis (5.2 and 5.3 percent, respectively) compared with those on placebo (3.1 and 1.6 percent). In a related editorial 62 in JCO, Drs. Nancy U. Lin and Eric P. Winer from Dana-Farber Cancer Institute noted that there is a chance that the findings could be understating those comorbid effects. There is a subset of patients treated with aromatase inhibitors "who experience life-altering arthralgias or other adverse effects, leading to difficult discussions weighing [quality of life] against recurrence risk," they wrote.
Everolimus Extends Progression-Free Survival in Advanced Kidney Cancer
A 400-patient, international phase III trial 63 testing the drug everolimus in patients with advanced kidney cancer has been stopped after meeting its primary endpoint, the drug's manufacturer, Novartis, reported February 28.
The trial's Independent Data Monitoring Committee recommended that the trial be halted and patients in the placebo arm of the study be offered everolimus after an interim analysis showed a significant improvement in progression-free survival in patients given everolimus.
Everolimus, also known as RAD001, inhibits mTOR, a protein that regulates tumor cell division and angiogenesis.
Patients in this cancer trial had advanced, progressive disease despite treatment with other recently approved agents for advanced kidney cancer, including sorafenib 64 (Nexavar) and sunitinib 65 (Sutent). Trial participants may also have been treated with bevacizumab 66 (Avastin) and interferon.
Complete interim results from the trial, dubbed RECORD-1, will be presented in May at the American Society of Clinical Oncology annual meeting in Chicago, Novartis explained.
Protein May Control Spread of Breast Cancer
A single protein may trigger the spread of breast cancer cells to other parts of the body by altering the behavior of large numbers of genes. The protein, SATB1, could potentially be used to identify women at risk of metastasis and may be a therapeutic target, Dr. Terumi Kohwi-Shigematsu of the Lawrence Berkeley National Laboratory and her colleagues report in the March 13 Nature 67.
They describe the protein as a "genome organizer." It coordinates the activity of genes from different chromosome regions by changing how DNA is packaged in the cell nucleus. For cells to acquire new functions, such as invading other tissues, they may need to induce global changes in gene activity.
In breast cancer cells, SATB1 activity may be necessary for and sufficient to induce metastasis. Blocking the protein in an aggressive breast cancer cell line altered the activity of more than a thousand genes and caused the cells to stop tumor growth and metastasis. Conversely, expressing the protein in a non-aggressive cell line fueled growth and metastasis.
The researchers say that SATB1 activity in breast cancer cells could identify women with early-stage disease who are at risk of metastasis. The primary prognostic indicator of metastasis has been the involvement of the lymph nodes, but additional markers are needed.
"Once this protein is expressed in breast cancer cells there is a very high propensity for metastasis," said Dr. Kohwi-Shigematsu. "And it may be possible to predict poor prognosis based on SATB1 expression independently from lymph node status."
SATB1 activity has also been found in colon and lung tumors, and the researchers are investigating how the protein is activated.
In a step toward therapies, the researchers are investigating ways to deliver SATB1 inhibitors directly into breast cancer cells. This is necessary to avoid disrupting the protein's critical role in the development of certain immune cells.
Methylation Markers Suggest Recurrence Risk in Lung Cancer
The switching on and off of a specific set of genes, or methylation, in patients with stage I non-small-cell lung cancer (NSCLC) appears to increase the risk of recurrence following surgery, researchers are reporting. In fact, when two of these genes were methylated (or switched off) in tissue samples from the tumor and mediastinal lymph nodes, the recurrence risk increased as much as 25-fold.
In a small, single institution, nested case-control study published in the March 13 New England Journal of Medicine 68, Dr. Malcolm Brock, an associate professor of surgery at Johns Hopkins' Sidney Kimmel Comprehensive Cancer Center, and colleagues analyzed the methylation status of seven genes in tumor and lymph node samples from patients who had undergone surgery for stage I NSCLC. Previous research has suggested that these genes may be associated with NSCLC recurrence.
As many as 40 percent of patients with stage I NSCLC treated with surgery will die of recurrent disease, and approximately 80 percent of patients will experience recurrence within 40 months.
Samples from 51 patients whose disease had recurred within 40 months (cases) were compared with samples from 116 patients whose cancers had not recurred (controls). The findings were tested against a small validation cohort of 11 cases and 9 controls.
The methylation status of four of the seven genes - p16, CDH13, RASSF1A, and APC - in tumor and/or lymph node samples were associated with varying levels of increased recurrence risk. The most lethal gene methylation combination was p16 and CDH13. In the original cohort, the 5-year recurrence-free survival rate was 14.3 percent in patients with methylation-induced silencing of both genes in the tumor and mediastinal lymph nodes (in the center of the chest, between the lungs), compared to 63.1 percent in patients without methylation of either gene.
"The DNA evidence we see for many of the recurring cases suggests it may be wise, if our work is confirmed, to reclassify such cancers as advanced disease instead of early stage," Dr. Brock said.
Elderly Medicaid Patients Less Likely to Receive Chemotherapy for Colorectal Cancer
A study using data from the Michigan Tumor Registry and the Centers for Medicare and Medicaid Services showed that elderly Medicaid-insured patients in the state are less likely to initiate or complete chemotherapy for colorectal cancer compared with Medicare-insured patients. The results were published in the March 10 Archives of Internal Medicine 69. Previous studies have shown that Medicaid-insured patients have worse survival rates for colorectal cancer, but it had not been known if they receive less treatment than patients with other forms of insurance.
The investigators collected data from 4,765 patients aged 65 or older who were diagnosed with colorectal cancer between January 1997 and December 2000 and insured through Medicaid, Medicare, or both. In addition to data on chemotherapy initiation and completion, the investigators compared whether patients were evaluated by an oncologist, subsequently hospitalized, and experienced comorbidities; demographic variables including age, race, sex, household income, and whether patients lived in a metropolitan, urban, or rural area were also studied.
Patients insured through Medicaid were more likely to be African American or of another minority race, female, and to live in a low-income area. For all patients, those with Medicaid insurance were less likely to initiate or complete chemotherapy and less likely to be evaluated by a medical oncologist. Older patients in general were also less likely to initiate chemotherapy, even though studies have shown that these patients benefit from adjuvant treatment.
"The main finding of this study is that Medicaid insurance is associated with a low likelihood of chemotherapy initiation and completion," concluded the authors. "As long as these substantially large groups of patients…have disparate treatment uptake and compliance, the nation as a whole will have difficulty reaching its goals for reduced cancer mortality."
|
 |
|
Table of Links
| 1 | http://www.cancer.gov/cancertopics/pdq/treatment/osteosarcoma |
| 2 | http://www.cancer.gov/cancertopics/treatment/childhoodcancers |
| 3 | http://www.childrensoncologygroup.org |
| 4 | http://home.ccr.cancer.gov/oncology/pediatric |
| 5 | http://web.ncifcrf.gov/atp |
| 6 | http://ccr.cancer.gov/research/ostp |
| 7 | http://cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_031808/page2 |
| 8 | http://www.cancer.gov/cancertopics/pdq/treatment/ewings/HealthProfessional/page1 |
| 9 | http://www.cancer.gov/clinicaltrials/2007-0515 |
| 10 | http://www.cancer.gov/cancertopics/types/childrhabdomyosarcoma |
| 11 | http://cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_031808/page3 |
| 12 | http://clinicalcenter.nih.gov |
| 13 | http://seer.cancer.gov |
| 14 | http://dccps.nci.nih.gov/ocs |
| 15 | http://www.livestrong.org |
| 16 | http://cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_031808/page9 |
| 17 | http://www.childrensinn.org |
| 18 | http://www.cancer.gov/cancertopics/factsheet/ALLinchildren |
| 19 | http://dceg.cancer.gov |
| 20 | http://www.cancer.gov/cancertopics/types/leukemia |
| 21 | http://ije.oxfordjournals.org/cgi/content/full/dyl299 |
| 22 | http://www.ncbi.nlm.nih.gov/pubmed/17684144?ordinalpos=1&itool=EntrezSystem
2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum |
| 23 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_112106/page3 |
| 24 | http://ctep.cancer.gov |
| 25 | http://www.cancer.gov/clinicaltrials/COG-ANUR0532 |
| 26 | http://www.cancer.gov/clinicaltrials/NCCAM-02-AT-0172 |
| 27 | http://www.cancer.gov/clinicaltrials/COG-ACCL05C1 |
| 28 | http://researchportfolio.cancer.gov |
| 29 | http://cancergenome.nih.gov |
| 30 | http://www.cancer.gov/cancertopics/types/neuroblastoma |
| 31 | http://pptp.stjude.org |
| 32 | http://www.ncbi.nlm.nih.gov/pubmed/18260118?ordinalpos=3&itool=EntrezSystem
2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum |
| 33 | http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&T
ermToSearch=18085673&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel
.Pubmed_RVDocSum |
| 34 | http://ccr.nci.nih.gov |
| 35 | http://www.cancer.gov/cancertopics/types/ewing |
| 36 | http://meeting.ascopubs.org/cgi/content/abstract/25/18_suppl/9560 |
| 37 | http://planning.cancer.gov/disease/AYA-Snapshot.pdf |
| 38 | http://planning.cancer.gov/disease/AYAO_PRG_Report_2006_FINAL.pdf |
| 39 | http://cancercontrol.cancer.gov |
| 40 | http://planning.cancer.gov/disease/Lymphoma-Snapshot.pdf |
| 41 | http://planning.cancer.gov/disease/Sarcoma-Snapshot.pdf |
| 42 | http://www.abstracts2view.com/hem07/view.php?nu=HEM07L1_3702 |
| 43 | http://www.cancer.gov/clinicaltrials/CALGB-10403 |
| 44 | http://www.nap.edu/catalog.php?record_id=11993 |
| 45 | http://www.apos-society.org/professionals/tools-resources/handbook/pediatric/pe
diatrichandbook.aspx |
| 46 | http://www.cancer.gov/cancertopics/coping/ccss |
| 47 | http://www.ncbi.nlm.nih.gov/pubmed/17035650 |
| 48 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_060606/page9 |
| 49 | http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_091107/page5 |
| 50 | http://cancercontrol.cancer.gov/ocs |
| 51 | http://cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_030408/page3#c |
| 52 | http://cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_051507/page2 |
| 53 | http://www.nih.gov/news/health/mar2008/niaid-12.htm |
| 54 | http://www.cancer.gov/cancertopics/druginfo/cetuximab |
| 55 | http://www.ncbi.nlm.nih.gov/pubmed/18337601 |
| 56 | http://www.ncbi.nlm.nih.gov/pubmed/18319413 |
| 57 | http://www.ncbi.nlm.nih.gov/pubmed/18319420 |
| 58 | http://www.cancer.gov/cancertopics/druginfo/letrozole |
| 59 | http://www.cancer.gov/cancertopics/druginfo/tamoxifencitrate |
| 60 | http://www.cancer.gov/newscenter/pressreleases/letrozole |
| 61 | http://www.ncbi.nlm.nih.gov/pubmed/18332475 |
| 62 | http://www.ncbi.nlm.nih.gov/pubmed/18332468 |
| 63 | http://www.cancer.gov/clinicaltrials/CRAD001C2240 |
| 64 | http://www.cancer.gov/cancertopics/druginfo/sorafenibtosylate |
| 65 | http://www.cancer.gov/cancertopics/druginfo/sunitinibmalate |
| 66 | http://www.cancer.gov/cancertopics/druginfo/bevacizumab |
| 67 | http://www.ncbi.nlm.nih.gov/pubmed/18337816 |
| 68 | http://www.ncbi.nlm.nih.gov/pubmed/18337602 |
| 69 | http://www.ncbi.nlm.nih.gov/pubmed/18332299 |
|
 |