Premarket Approval Information - Devices
HIV 1/2 STAT-PAKT ASSAY
20 Tests
| Read this Product Insert completely before using the product. Follow the instructions carefully when performing the test as not doing so may result in inaccurate Test Results. Users of this test should follow the CDC Universal Precautions for prevention of transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other blood borne pathogens. [1] |
COMPLEXITY: MODERATE
STORAGE: Store at 8 to 30°C (46 to 86°F)
NAME AND INTENDED USE
The Chembio HIV 1/2 STAT-PAKT Assay is a single-use immunochromatographic test for the detection of
antibodies to Human Immunodeficiency Virus Type 1 (HIV-1) and Type 2 (HIV-2) in fingerstick whole blood, venous whole blood, serum or plasma specimens. The Chembio HIV 1/2 STAT-PAKT assay is intended for use as a point-of-care test to aid in the diagnosis of infection with HIV-1 and HIV-2. This test is suitable for use in multi-test algorithms designed for the statistical validation of rapid HIV test results. When multiple rapid HIV tests are available, this test should be used in appropriate multi-test algorithms.
RESTRICTIONS
- Sale of the Chembio HIV 1/2 STAT-PAKT Assay is restricted to clinical laboratories that have an adequate quality assurance program, including planned systematic activities that provide adequate confidence that requirements for quality will be met and where there is assurance that operators will receive and use the instructional materials.
- The Chembio HIV 1/2 STAT-PAKT Assay is approved for use only by an agent of a clinical laboratory.
- Test subjects must receive the "Subject Information Notice" prior to specimen collection and appropriate information when test results are provided.
- The Chembio HIV 1/2 STAT-PAKT Assay is not approved for use to screen blood, plasma, cell or tissue donors.
SUMMARY AND EXPLANATION OF THE TEST
Discovered in 1983, the Human Immunodeficiency Virus is a retrovirus and identified as the etiologic agent for the Acquired Immunodeficiency Syndrome (AIDS), and AIDS related complex [2]. AIDS is characterized by changes in the population of T-cell lymphocytes that play a key role in the immune defense system. In the infected individual the virus causes a depletion of a subpopulation of T-cells, called T-helper cells, which leaves these patients susceptible to opportunistic infections and certain malignancies. The major routes of transmission are sexual contact, exposure to contaminated blood or blood products (including sharing of contaminated syringes and needles) and mother-tonewborn transmission [3-5].
Although there has been a decrease in the rate of infection in certain countries, the number of persons infected with HIV globally has continued to increase. By the end of 2005 there were approximately 40.3 million people living with HIV/AIDS, an increase from nearly 37.5 million in 2003. An estimated 5 million people were newly infected with HIV in 2005. In the same year more than 3 million died of AIDS-related illness; more than 500,000 of these were children [6].
HIV infection, AIDS and AIDS related complex have become a leading cause of illness and death in the United States for the past two decades. As of December 2001, a total of 774,467 persons were reported with AIDS and 448,060 of these persons had died. Approximately 800,000 - 900,000 persons in the United States are infected with HIV and approximately 80,000 - 280,000 of these persons may not be aware of their infected status [7].
The HIV virus consists of a genomic RNA molecule protected by a capsid and an envelope. The HIV envelope is the major target for a humoral antibody response. The presence of the virus in patients causes the immune system to elicit the production of antibodies. The detection of these antibodies can be used as a diagnostic tool.
Enzyme Immunoassays (EIAs), Western Blots (WB), Nucleic Amplification Test (NAT) assay and various other test systems are currently available for detection of HIV-1 and HIV-2 infection [8-12]. The Chembio HIV 1/2 STAT-PAKT Assay utilizes immobilized antigens for the detection of antibodies to HIV-1 and HIV-2, and is a point-of-care test to aid in the diagnosis of infection with HIV-1 and HIV-2.
BIOLOGICAL PRINCIPLES OF THE TEST
The Chembio HIV 1/2 STAT-PAKT Assay employs a unique combination of a specific antibody binding protein
which is conjugated to colloidal gold dye particles and HIV-1/2 antigens which are bound to the solid phase
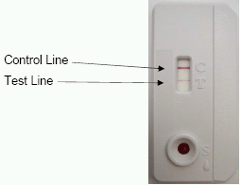
membrane. The venous or capillary (fingerstick) whole blood, serum or plasma is applied to the SAMPLE (S) well of test device followed by the addition of Running Buffer. The Buffer facilitates the lateral flow of the specimen and test reagents and promotes the binding of the antibodies to the antigen. The specimen/buffer mixture migrates along the test strip by capillary action, reconstituting the conjugate. If present, the antibodies bind to the colloidal gold conjugated antibody binding protein. In a reactive sample, the dye conjugated-immune complex migrates on the nitrocellulose membrane and is captured by the antigens immobilized in the TEST (T) area producing a pink/purple line. In the absence of HIV-1 and HIV-2 antibodies, there is no pink/purple line in the TEST (T) area. The sample continues to migrate along the membrane and produces a pink/purple line in the CONTROL (C) area containing immunoglobulin G antigens. This procedural control serves to demonstrate that specimen and reagents have been properly applied and have migrated through the device.
MATERIALS PROVIDED
Each Kit contains the components to perform 20 tests:
-
20 STAT-PAKT Individually Pouched Test Devices
20 Copies of Subject Information Notice
20 Disposable 5µL Sample Loops
1 HIV Running Buffer (5mL)
1 Product Insert for the HIV 1/2 STAT-PAKT Assay
MATERIALS REQUIRED AND AVAILABLE AS AN ACCESSORY TO THE KIT
Chembio HIV Reactive/Nonreactive Controls (Catalog # HIV104).
Each package contains:
-
1 HIV 1 Reactive Control (0.5mL)
1 HIV 2 Reactive Control (0.5mL)
1 Nonreactive Control (0.5mL)
1 Product Insert for Catalog #HIV104
MATERIALS REQUIRED BUT NOT PROVIDED
- Clock, watch, or other timing device
- Pipettor capable of delivering 5µL of sample may be used in lieu of the disposable 5µL sample loop supplied with the Kit (for other than fingerstick whole blood specimens)
- Disposable gloves
- Sterile gauze (for fingerstick whole blood specimens)
- Antiseptic wipes
- Biohazard disposal container
- Sterile Safety Lancet (for fingerstick whole blood specimens)
- Collection devices for specimens (other than fingerstick whole blood specimens)
WARNINGS
For IN VITRO diagnostic use
- Read the Product Insert completely before using this assay. Follow the instructions carefully as not doing so may result in inaccurate Test Results.
- Users of this test should follow the CDC Universal Precautions for prevention of transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other bloodborne pathogens. [1]
- Use of this test Kit with specimen types other than those specifically approved for use with this device may result in inaccurate test results.
- This test should be performed at 18 to 30°C (64 to 86°F). If stored refrigerated, ensure that the pouch is brought to operating temperature before performing testing.
- If the test Kit is stored at temperatures outside the storage temperature 8 to 30°C (46 to 86°F), or used outside the operating temperature 18 to 30°C (64 to 86°F), use the Kit Controls to ensure proper performance of the test.
- Individual infected with HIV-1 and/or HIV-2 who is receiving highly active antiretroviral therapy (HAART) may produce false negative results.
PRECAUTIONS
Safety Precautions
- Handle the specimens and materials contacting specimens as if capable of transmitting infection.
- Do not eat, drink or smoke in the area where specimens and kit reagents are handled. Avoid any contact with hands, eyes or mouth during specimen collection and testing.
- Wear protective clothing such as laboratory coats, disposable gloves and eye protection when handling specimens.
- Dispose of all specimens and materials used in the test procedure in a biohazard waste container. Lancets should be placed in a puncture-resistant container prior to disposal. The recommended method of disposal of biohazard waste is autoclaving for a minimum of 1 hour at 121ºC. Disposable materials may be incinerated. Liquid wastes may be mixed with appropriate chemical disinfectants. A freshly prepared solution of 10% bleach (0.5% solution of sodium hypochlorite) is recommended. Allow 60 minutes for effective decontamination. NOTE: Do not autoclave solutions that contain bleach.
- For additional information on biosafety, refer to "Universal Precautions for prevention of transmission of Human Immunodeficiency Virus, Hepatitis B Virus, and other bloodborne pathogens." [1] And "Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposure to HBV, HCV, and HIV and Recommendations for Postexposure Prophylaxis [13]
- Use 10% bleach or other appropriate disinfectant to wipe all spills. The bleach solution should be made fresh each day.
Handling Precautions
- If Desiccant Packet is missing, DO NOT USE, discard test device, and a new test device should be used.
- Do not use any test device if the pouch has been perforated.
- Each test device is for single use only.
- Do not use the reagents beyond the expiration date printed on the pouch. Always check expiration date prior to testing.
- Do not mix reagents from different lot numbers of Kits.
- Adequate lighting is required to read the Test Results.
STORAGE
The Chembio HIV 1/2 STAT-PAKT Assay should be stored in its unopened pouch at 8 to 30°C (46 to 86°F). Do not freeze. Do not open the pouch until you are ready to perform a test. When stored as indicated, test devices are stable until the expiration date marked on the pouch. Running Buffer should also be stored at 8 to 30°C (46 to 86°F) in its original vial.
SPECIMEN COLLECTION
Prior to specimen collection, provide test subjects with Subject Information Notice and pre test counselingaccording to CDC Guidelines for Rapid HIV Testing [7].
The Chembio HIV 1/2 STAT-PAKT Assay is performed on fingerstick whole blood, venous whole blood, serum or plasma specimens.
Fingerstick Whole Blood: Prepare to perform the fingerstick blood collection procedure. Clean the finger of the person being tested with an antiseptic wipe. Allow the finger to dry thoroughly or wipe dry with a sterile gauze pad. Using a sterile lancet, puncture the skin just off the center of the finger and wipe away the first drop with sterile gauze and avoid squeezing the fingertip to accelerate bleeding as this may dilute the blood with excess tissue fluid. Collect the sample from the second drop touching the disposable Sample Loop provided to the drop of blood until the Sample Loop is full. Test immediately, following Test Procedure Instructions.
Venous Whole Blood: Draw blood following laboratory procedures for obtaining venous blood. Collect sample in a tube containing citrate, heparin, or EDTA. Be sure the tube of blood is well mixed.
Serum or Plasma: Draw blood following laboratory procedures for obtaining serum or plasma specimens. Collect specimen in a tube not containing any anticoagulant (serum), and in a tube containing citrate, heparin, or EDTA (plasma). Collect specimen in a clean container following standard laboratory procedures.
Venous whole blood, serum and plasma specimens may be tested immediately after collection. If specimens are not tested immediately, refrigerate them at 2 to 8°C (36 to 46°F) following collection. These specimens should be tested within 3 days of collection. If specimens are not tested within 3 days of collection, serum or plasma specimens should be frozen at -20°C (-4°F) or colder. DO NOT FREEZE WHOLE BLOOD!
Specimen Shipping
If specimens are to be shipped, they should be packed in compliance with regulations covering the transportation of etiologic agents. Venous whole blood, serum and plasma specimens should be shipped refrigerated with cold packs or wet ice.
TEST PROCEDURE
Kit Component Preparation
All components for the Chembio HIV 1/2 STAT-PAKT Assay test are ready to use as supplied. Follow directions as indicated. If the specimen to be tested is refrigerated, remove it from the refrigerator and allow it to come to a temperature of 18 to 30°C: (64 to 86°F) prior to testing.
|
Figure 1
|
|
Figure 2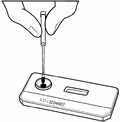 |
|
Figure 3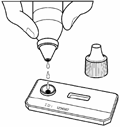 |
|
|
QUALITY CONTROL
Built-in Control Feature
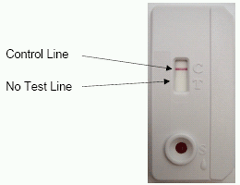
The control line serves as a built-in internal control and gives confirmation of sample addition and proper test performance. A pink/purple line will appear in the CONTROL (C) area if the test has been performed correctly and the device is working properly (Please see section: Interpretation of Test Results).
External Quality Control
Good Laboratory Practices (GLP) necessitates testing external control material along with the test samples to ensure proper performance of the test Kit. Chembio HIV Reactive/Nonreactive Controls (Catalog # HIV104) are available separately for use with the Chembio HIV 1/2 STAT-PAKT Assay. The HIV Controls are used to verify the operator's ability to properly perform the test and to interpret the results. Each Reactive Control will produce a REACTIVE Test Result and has been manufactured to produce a faint line in the TEST (T) area. The Nonreactive Control will produce a NONREACTIVE Test Result. Run the Controls as per the TEST PROCEDURE and follow the instructions as described in INTERPRETATION OF TEST RESULTS sections of this Product Insert. It is the responsibility of each facility using the Chembio HIV 1/2 STAT-PAKT assay to establish an adequate quality assurance program to ensure the performance of the device under specific locations and conditions of use.
Run the Kit Controls under the following circumstances:
If the HIV Control reagents do not produce the expected results contact Chembio Diagnostic Systems Customer Service either at 1-631-924-1135 or, Toll Free at 1-800-327-3635.
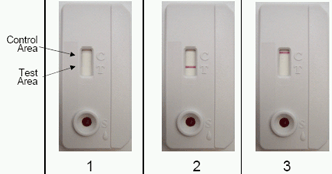
INTERPRETATION OF TEST RESULTS
|
|
|
LIMITATIONS OF THE PROCEDURE
- The Chembio HIV 1/2 STAT-PAKT test must be used in accordance with the instructions in this Product Insert to obtain accurate results.
- The Chembio HIV 1/2 STAT-PAKT Assay must be used with capillary (fingerstick) or venous whole blood, serum or plasma only. Use of other types of specimens or testing of venipuncture whole blood specimens collected using a tube containing an anticoagulant other than citrate, heparin or EDTA may not yield accurate results. For serum samples, collect blood without anticoagulant.
- Reading Nonreactive Test Results earlier than 15 minutes or any Test Results later than 20 minutes may yield erroneous results.
- Do not open the sealed foil pouch until just prior to use.
- Do not use Kit contents beyond labeled expiration date.
- For the collection of the fingerstick whole blood specimen, ensure that finger is completely dry before performing fingerstick.
- Read results in a well-lit area.
- A Reactive Test Result using the Chembio HIV 1/2 STAT-PAKT test suggests the presence of antibodies to HIV-1 and/or HIV-2 in the specimen. The Chembio HIV 1/2 STAT-PAKT Assay is intended as an aid in the diagnosis of infection with HIV-1/2. AIDS and AIDS-related conditions are clinical syndromes and their diagnosis can only be established clinically.
- For a Reactive Test Result, the intensity of the test line does not necessarily correlate with the titer ofantibody in the specimen.
- A person who has antibodies to HIV-1 or HIV-2 is presumed to be infected with the virus, except that a person who has participated in an HIV vaccine study may develop antibodies to the vaccine and may or may not be infected with HIV.
- A Nonreactive Test Result does not preclude the possibility of exposure to HIV or infection with HIV. An antibody response to recent exposure may take several months to reach detectable levels.
- This assay has not been evaluated for newborn screening, cord blood specimens, individuals less than 18 years and greater than 64 years of age.
PERFORMANCE CHARACTERISTICS
The Chembio HIV 1/2 STAT-PAKT Assay was evaluated in prospective clinical studies at four geographically
distinct sites. The specimens were tested from three groups of individuals: Known infected with HIV-1, at high risk for infection with HIV-1, and at low risk for infection with HIV-1. The Chembio HIV 1/2 STAT-PAKT Assay was tested in parallel on fingerstick whole blood, venous whole blood, serum and plasma specimen matrices. No discordant result was obtained from all specimen matrices. The serum/plasma specimens from study subjects were also tested using a licensed Enzyme Immunoassay (EIA). Specimens with discordant results were further tested using licensed Western Blot and/or FDA approved NAT assay.
SENSITIVITY
The sensitivity of the Chembio HIV 1/2 STAT-PAKT Assay to detect infection with HIV-1 was evaluated using 603 specimens from individuals known to be infected with HIV-1 and from 776 individuals at high risk for infection with HIV-1 (Table 1). 637 individuals were identified as positive for infection with HIV-1 using a licensed confirmatory Assay, and/or FDA approved NAT assay. Of these, 635 tested reactive on the Chembio HIV 1/2 STAT-PAKT Assay. The calculated sensitivity of Chembio HIV 1/2 STAT-PAKT assay in these studies was 99.7% (635/637 = 99.5% with 95% CI = 98.9% - 100%).
| Study Population | Samples | HIV 1/2 STATPAK™ Assay Reactive |
Licensed EIA Repeatedly Reactive |
Licensed WB Reactive |
True Positive 1 |
|---|---|---|---|---|---|
| Known Positive | 603 | 599 | 601 | 601 | 601 |
| HighRisk | 776 | 36 | 41 | 352 | 363 |
| TOTAL | 1379 | 635 | 642 | 636 | 637 |
2 Two specimens were indeterminate by Western Blot (one was initially reactive only on EIA and Nonreactive on HIV 1/2 STAT-PAKT, and one was repeatedly reactive on EIA and Nonreactive on HIV 1/2 STAT-PAKT).
3 One specimen was repeatedly reactive on EIA and Reactive on HIV 1/2 STAT-PAKT, indeterminate on WB, and positive on NAT.
The Sensitivity of the Chembio HIV 1/2 STAT-PAKT Assay to detect HIV-antibody was determined by testing 202 serum/plasma specimens that were positive for HIV-2 antibodies only. These specimens were obtained from repository sources. A total of 488 specimens from an area endemic for HIV-infection were also tested (Table 2). All specimens reactive with the Chembio HIV 1/2 STAT-PAKT in these studies were also reactive by a licensed anti-HIV-1/2 EIA. The sensitivity of HIV 1/2 STAT-PAKT for detection of antibodies to HIV-2 in these studies was calculated to be 100% (203/203 = 100% with 95% CI = 98.2% - 100%).
| Study Population | Samples | HIV 1/2 STATPAK™ Assay Reactive |
True HIV2 Positive Only 1 |
|---|---|---|---|
| Known HIV2 Positive | 202 | 202 | 202 |
| Endemic Samples | 488 | 272 | 1 |
| TOTAL | 690 | 229 | 203 |
2 Of these 27 HIV-1 EIA and HIV-2 EIA reactive specimens; 23 were reactive on HIV-1 WB only, three were reactive on HIV-1 and HIV-2 WB, and one was indeterminate on HIV-1 WB and reactive on HIV-2 WB.
REACTIVITY WITH HIV-1 SPECIMENS FROM VARIOUS WORLD WIDE GEOGRAPHIC REGIONS
To assess sensitivity of the Chembio HIV 1/2 STAT-PAKT Assay for HIV-1 specimens from various worldwide geographic regions, 1,894 confirmed HIV antibody-positive specimens were obtained. Of the 1,859 specimens evaluated from Africa, Asia, Latin America, Europe, and Belgium, 1,854 were reactive using Chembio HIV 1/2 STAT-PAKT Assay. The remaining 35 confirmed positive specimens, from two worldwide panels were evaluated, consisting of naturally occurring plasma specimens from diverse geographical locations (Spain, Ghana, Cote d' Ivoire, Mozambique, Uganda, Zimbabwe, China, Thailand, India, USA and Argentina with HIV-genotypes: A, B, C, D, E, F, G, O, B/D and HIV-2). All 35 were reactive using Chembio HIV 1/2 STAT-PAKT Assay.
REACTIVITY WITH SEROCONVERSION PANELS
The Chembio HIV 1/2 STAT-PAKT Assay was tested against 15 different seroconversion panels. Each panel consisted of sequential collections from single individual who seroconverted. Samples were confirmed using Western Blot (WB) and two licensed EIA tests. As shown in Table 3, the Chembio HIV 1/2 STAT-PAKT test performed similarly to currently licensed assays in detecting seroconversion.
| Panel | Relative Day of Bleed |
Chembio HIV 1/2 STAT PAK™ Assay |
EIA 1 | EIA 2 | WB |
|---|---|---|---|---|---|
| PRB-927 (AB) | 0 | NR | NR | NR | NR |
| 28 | NR | RR | NR | NR | |
| 33 | NR | RR | NR | NR | |
| 35 | R | RR | NR | NR | |
| 40 | R | RR | RR | R | |
| PRB-928 (AC) | 0 | NR | NR | NR | NR |
| 111 | NR | RR | NR | NR | |
| 120 | R | RR | RR | R | |
| 125 | R | RR | RR | R | |
| 130 | R | RR | RR | R | |
| PRB-930 (AE) | 0 | NR | NR | NR | NR |
| 3 | NR | NR | NR | NR | |
| 7 | NR | RR | NR | NR | |
| 10 | NR | RR | RR | IND | |
| PRB-931 (AF) | 0 | NR | NR | NR | NR |
| 2 | NR | NR | NR | NR | |
| 7 | NR | NR | NR | NR | |
| 9 | NR | NR | NR | NR | |
| 15 | NR | NR | NR | NR | |
| 28 | R | RR | NR | NR | |
| 33 | R | RR | RR | IND | |
| 35 | R | RR | RR | R | |
| 42 | R | RR | RR | R | |
| PRB-934 (AI) | 0 | NR | NR | NR | NR |
| 7 | R | RR | NR | D | |
| 11 | R | RR | RR | IND | |
| PRB-938 (AM) | 0 | NR | NR | NR | NR |
| 3 | NR | NR | NR | NR | |
| 9 | NR | RR | NR | IND | |
| PRB-944 (AT) | 0 | NR | NR | NR | NR |
| 2 | NR | NR | NR | NR | |
| 7 | NR | NR | NR | NR | |
| 9 | NR | NR | NR | NR | |
| 14 | R | RR | NR | IND | |
| 16 | R | RR | NR | IND | |
| PRB-959 (BI) | 0 | NR | NR | NR | NR |
| 7 | NR | NR | NR | NR | |
| 9 | NR | RR | NR | NR | |
| 14 | R | RR | RR | R | |
| 19 | R | RR | RR | R | |
| 21 | R | RR | RR | R | |
| 26 | R | RR | RR | R |
| Panel | Relative Day of Bleed |
Chembio HIV 1/2 STAT PAK™ Assay |
EIA 1 | EIA 2 | WB |
|---|---|---|---|---|---|
| PRB-904 (D) | 0 | NR | NR | NR | NR |
| 21 | NR | NR | NR | NR | |
| 49 | NR | NR | NR | NR | |
| 92 | R | RR | RR | R | |
| 99 | R | RR | RR | R | |
| PRB-910 (J) | 0 | NR | NR | NR | NR |
| 14 | NR | NR | NR | NR | |
| 26 | R | RR | RR | R | |
| 28 | R | RR | RR | R | |
| 32 | R | RR | RR | R | |
| 35 | R | RR | RR | R | |
| 40 | R | RR | RR | R | |
| PRB-914 (N) | 0 | R | RR | NR | IND |
| 4 | R | RR | RR | IND | |
| 7 | R | RR | RR | IND | |
| 25 | R | RR | RR | R | |
| 31 | R | RR | RR | R | |
| PRB-916 (P) | 0 | NR | NR | NR | NR |
| 4 | NR | NR | NR | NR | |
| 9 | NR | NR | NR | NR | |
| 15 | NR | NR | NR | NR | |
| 30 | R | RR | RR | R | |
| 35 | R | RR | RR | R | |
| PRB-917 (Q) | 0 | NR | NR | NR | NR |
| 53 | NR | NR | NR | NR | |
| 57 | NR | NR | NR | NR | |
| 60 | N/A* | RR | NR | NR | |
| 65 | R | RR | NR | IND | |
| 67 | R | RR | NR | IND | |
| 72 | N/A | RR | RR | R | |
| PRB-919 (S) | 0 | NR | NR | NR | NR |
| 9 | R | RR | NR | R | |
| 11 | NR | RR | RR | R | |
| PRB-922 (V) | 0 | NR | RR | NR | NR |
| 4 | NR | RR | NR | NR | |
| 7 | R | RR | NR | NR | |
| 11 | R | RR | NR | R |
*N/A - Not Available.
REACTIVITY WITH HIV-1 LOW TITER PANELS
A total of 30 specimens from two characterized Low Titer panels were used to evaluate the ability of the Chembio HIV 1/2 STAT-PAKT Assay to detect antibodies to HIV-1. The results of testing are presented in Table 4 and demonstrate that the Chembio HIV 1/2 STAT-PAKT Assay detected the presence of antibody in a manner similarly to one or both of the licensed EIAs.
| Panel | Chembio HIV 1/2 STATPAK™ Assay |
EIA 1 | EIA 2 | WB |
|---|---|---|---|---|
| PRB107-1 | NR | RR | NR | NR |
| PRB107-2 | NR | RR | RR | IND |
| PRB107-3 | NR | RR | NR | NR |
| PRB107-4 | NR | RR | RR | NR |
| PRB107-5 | NR | NR | NR | NR |
| PRB107-6 | R | RR | RR | NR |
| PRB107-7 | NR | RR | NR | NR |
| PRB107-8 | NR | RR | RR | NR |
| PRB107-9 | NR | RR | NR | NR |
| PRB107-10 | R | RR | RR | NR |
| PRB107-11 | R | RR | RR | R |
| PRB107-12 | NR | RR | NR | NR |
| PRB107-13 | NR | RR | NR | IND |
| PRB107-14 | R | RR | RR | R |
| PRB107-15 | R | RR | RR | IND |
| PRB108-1 | R | RR | RR | R |
| PRB108-2 | NR | NR | NR | NR |
| PRB108-3 | NR | RR | RR | IND |
| PRB108-4 | R | RR | RR | R |
| PRB108-5 | R | RR | RR | R |
| PRB108-6 | R | RR | RR | IND |
| PRB108-7 | R | RR | RR | R |
| PRB108-8 | R | RR | RR | R |
| PRB108-9 | R | RR | RR | R |
| PRB108-10 | NR | RR | NR | IND |
| PRB108-11 | R | RR | RR | R |
| PRB108-12 | NR | RR | NR | NR |
| PRB108-13 | NR | RR | NR | IND |
| PRB108-14 | NR | RR | NR | NR |
| PRB108-15 | R | RR | RR | IND |
REACTIVITY WITH HIV-1 MIXED TITER PANELS
The sensitivity of Chembio HIV 1/2 STAT-PAKT Assay was evaluated by testing three well characterized panels composed of specimens ranging from nonreactive to strong reactive for anti-HIV-1 antibody. The results are presented in Table and indicate that the Chembio HIV 1/2 STAT-PAKT Assay was able to detect antibodies to HIV-1 similarly to the licensed EIA and WB.
| Panel | Chembio HIV 1/2 STATPAK™ Assay |
EIA 1 | EIA 2 | WB |
|---|---|---|---|---|
| PRB202-1 | NR | RR | RR | IND |
| PRB202-2 | R | RR | RR | R |
| PRB202-3 | R | RR | RR | R |
| PRB202-4 | R | RR | RR | R |
| PRB202-5 | R | RR | RR | R |
| PRB202-6 | R | RR | RR | R |
| PRB202-7 | R | NR | RR | R |
| PRB202-8 | R | RR | RR | R |
| PRB202-9 | NR | NR | NR | NR |
| PRB202-10 | R | RR | RR | R |
| PRB202-11 | R | RR | RR | R |
| PRB202-12 | R | RR | RR | R |
| PRB202-13 | NR | RR | RR | R |
| PRB202-14 | R | RR | RR | R |
| PRB202-15 | R | RR | RR | R |
| PRB202-16 | R | RR | RR | R |
| PRB202-17 | R | RR | RR | R |
| PRB202-18 | R | RR | RR | R |
| PRB202-19 | R | RR | RR | R |
| PRB202-20 | R | RR | RR | R |
| PRB202-21 | NR | NR | NR | NR |
| PRB202-22 | R | RR | RR | R |
| PRB202-23 | NR | RR | NR | IND |
| PRB202-24 | R | RR | RR | R |
| PRB202-25 | R | RR | RR | R |
| PRB203-1 | R | RR | RR | R |
| PRB203-2 | R | RR | RR | R |
| PRB203-3 | NR | NR | NR | NR |
| PRB203-4 | NR | RR | NR | IND |
| PRB203-5 | R | RR | RR | R |
| PRB203-6 | R | RR | RR | R |
| PRB203-7 | R | RR | RR | R |
| PRB203-8 | R | RR | RR | R |
| PRB203-9 | R | RR | RR | R |
| PRB203-10 | R | RR | RR | R |
| PRB203-11 | R | RR | RR | R |
| PRB203-12 | R | RR | RR | R |
| PRB203-13 | R | RR | RR | R |
| PRB203-14 | NR | RR | NR | NR |
| PRB203-15 | R | RR | RR | R |
| PRB203-16 | R | RR | RR | R |
| PRB203-17 | R | RR | RR | R |
| PRB203-18 | R | RR | RR | R |
| PRB203-19 | R | RR | RR | R |
| Panel | Chembio HIV 1/2 STATPAK™ Assay |
EIA 1 | EIA 2 | WB |
|---|---|---|---|---|
| PRB203-20 | NR | NR | NR | NR |
| PRB203-21 | R | RR | RR | R |
| PRB203-22 | R | RR | RR | R |
| PRB203-23 | R | RR | RR | R |
| PRB203-24 | R | RR | RR | R |
| PRB203-25 | R | RR | RR | R |
| PRB204-1 | NR | RR | NR | NR |
| PRB204-2 | R | RR | RR | R |
| PRB204-3 | NR | NR | NR | NR |
| PRB204-4 | R | RR | RR | R |
| PRB204-5 | R | RR | RR | R |
| PRB204-6 | R | RR | RR | R |
| PRB204-7 | R | RR | RR | R |
| PRB204-8 | R | RR | RR | R |
| PRB204-9 | NR | RR | NR | NR |
| PRB204-10 | R | RR | RR | IND |
| PRB204-11 | R | RR | RR | R |
| PRB204-12 | R | RR | RR | R |
| PRB204-13 | NR | RR | RR | IND |
| PRB204-14 | R | RR | RR | R |
| PRB204-15 | R | RR | RR | R |
| PRB204-16 | R | RR | RR | R |
| PRB204-17 | R | RR | RR | R |
| PRB204-18 | R | RR | RR | IND |
| PRB204-19 | R | RR | RR | R |
| PRB204-20 | R | RR | RR | R |
| PRB204-21 | R | RR | RR | R |
| PRB204-22 | R | RR | RR | R |
| PRB204-23 | NR | NR | NR | NR |
| PRB204-24 | NR | NR | NR | IND |
| PRB204-25 | NR | NR | NR | IND |
SPECIFICITY
The specificity of Chembio HIV 1/2 STAT-PAKT Assay was evaluated by testing specimens from low risk and high risk populations for infection with HIV-1 from three clinical study sites. The results are summarized in Table 6.
| Study Population | Samples | HIV 1/2 STATPAK™ Assay Nonreactive |
Licensed EIA Nonreactive | True Negative 1 |
|---|---|---|---|---|
| LowRisk | 691 | 690 | 6872 | 691 |
| HighRisk | 776 | 740 | 7353 | 740 |
| TOTAL | 1467 | 1430 | 1422 | 1431 |
2 Four specimens were repeatedly reactive on EIA and nonreactive on Chembio HIV 1/2 STAT-PAKT Assay and Western Blot.
3 Five specimens were repeatedly reactive on EIA and nonreactive on Chembio HIV 1/2 STAT-PAKT Assay and Western Blot.
Based on these studies, the specificity of Chembio HIV 1/2 STAT-PAKT Assay in these studies was calculated to be 99.9% (1430/1431 = 99.9% with 95% CI = 99.6% - 100%).
EFFECT OF POTENTIALLY INTEFERING SUBSTANCES AND UNRELATED MEDICAL CONDITIONS
To evaluate the influence of unrelated medical conditions or interfering substance on the specificity and sensitivity of the Chembio HIV 1/2 STAT-PAKT Assay. 208 specimens representing unrelated medical conditions, and 110 specimens representing potential interfering substances were tested (Table 7). The specimens were spiked with either saline (Nonreactive) or an HIV-1 reactive serum specimen to a low level of reactivity. All HIV-1 spiked specimens gave reactive results while all unspiked samples, with the exception of one elevated albumin specimen and 14 syphilis specimens, gave nonreactive results. The one elevated albumin specimen and all of the 14 unspiked syphilis specimens with reactive results were subsequently confirmed as infected with HIV-1 using licensed Western Blot assay. An additional ten known HIV-1 nonreactive, syphilis reactive specimens were tested and yielded expected results.
| Chembio HIV 1/2 STATPAK™ Assay | ||
|---|---|---|
| Description | Saline (Nonreactive) | HIV-1/2 (Weak Reactive) |
| Cirrhosis | 20 / 20 | 20 / 20 |
| CMV IgM | 20 / 20 | 20 / 20 |
| Recent flu vaccination 1 | 11 / 11 | 11 / 11 |
| HBV | 21 / 21 | 21 / 21 |
| HCV | 19 / 19 | 19 / 19 |
| HTLV-I | 11 / 11 | 11 / 11 |
| HTLV-II | 10 / 10 | 10 / 10 |
| Multiparous | 9 / 9 | 9 / 9 |
| Myeloma | 10 / 10 | 10 / 10 |
| Rheumatoid Factor | 10 / 10 | 10 / 10 |
| Syphilis 2 | 15 / 29 | 29 / 29 |
| Tuberculosis | 38 / 38 | 38 / 38 |
| Elevated Albumin 3 | 9 / 10 | 10 / 10 |
| Elevated Bilirubin | 10 / 10 | 10 / 10 |
| Citrate | 10 / 10 | 10 / 10 |
| DNA | 10 / 10 | 10 / 10 |
| EDTA | 10 / 10 | 10 / 10 |
| Hemolyzed | 10 / 10 | 10 / 10 |
| Heparin | 10 / 10 | 10 / 10 |
| Icteric | 10 / 10 | 10 / 10 |
| Lipemic | 10 / 10 | 10 / 10 |
| Elevated Protein | 10 / 10 | 10 / 10 |
| Elevated Triglycerides | 10 / 10 | 10 / 10 |
2 Fourteen samples were confirmed reactive, using licensed WB assay
3 One sample was confirmed as containing HIV antibodies by using licensed WB assay
REPRODUCIBILITY STUDIES
Reproducibility was tested at three independent sites using three lots of Chembio HIV 1/2 STAT-PAKT Assay. A panel of five blinded samples representing nonreactive, low reactive HIV-1, low reactive HIV-2, high reactive HIV-and high reactive HIV-were run on three separate days by three separate technicians at each site. Testing was performed according to the Product Insert of the Chembio HIV 1/2 STAT-PAKT test. Results were read at 15 minutes. Results were read semi-quantitatively using common strip evaluation scale. total of 405 data points was taken. There was 100% reproducibility (405/405) across all parameters.
REFERENCES
- Centers for Disease Control and Prevention (CDC) Universal Precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health-care settings. MMWR 1988; 37(24):377-388.
- Essex, M. (1999) Human immunodeficiency viruses in the developing world. Adv Virus Res 53 : 71-88.
- Kanki, P.J., Hopper, J.R. and Essex, M. (1987) The origins of HIV-1 and HTLV-4/HIV/2. Ann N Y Acad Sci 511 370-375.
- Nicoll, A., Gill, O.N. (1999) The global impact of HIV infection and disease. Commun Dis Publ Health 2 85-95.
- Valdiserri R.O., Holtgrave, D.R., West, G.R. (1999) Promoting early diagnosis and entry into care. AIDS 13 2317-2330.
- UNAIDS / WHO Press Release (2005) HIV Infection Rates Decreasing in Several Countries but Globally Number of People Living with HIV Continue to Rise. Available at www.unaids.org/epi/2005/doc/docs/PR EpiUpdateNov05en.pdf: 1-3.
- CDC. Revised Guidelines for HIV Counseling, Testing and Referral and Revised Recommendations for HIV Screening of Pregnant Women. MMWR 2001; 50(19);32-35.
- Essex, M., Kanki, P. J., Marlink, R., et al. (1990) Antigenic characterization of the human immunodeficiency viruses. J Am Acad Dermatol 22 1206-1210.
- Essex, M., McLane, M.F., Lee, T.H. et al. (1983) Antibodies to cell membrane antigens associated with human T-cell leukemia virus in patients with AIDS. Science 220 859-862.
- Gallo, R.C., Saluahuddin, S.Z., Popovic, M., et al. (1984) Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 224 500-503.
- Kenealy, W., Reed, D., Cybulsky, R., et.al. (1987) Analysis of human serum antibodies to human immunodeficiency virus (HIV) using recombinant ENV and GAG antigens. AIDS Res Human Retrovir 3:95-105.
- Kovacs, A., Xu, J., Rasheed, S., et al. (1995) Comparison of a rapid non-isotopic polymerase chain reaction assay with four commonly used methods for the early diagnosis of human Immunodeficiency Virus type infection in neonates and children. Pediatr Infect Dis 14 948-954.
- CDC:Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HBV, HCV and Recommendations for Postexposure Prophylaxis. MMWR 2001; 50(RR-11):1-42.
- CDC: Approved of New Rapid Test for HIV Antibody. MMWR 1988; 37(24) 377-388.
Available Accessories:
HIV Reactive/Nonreactive Controls: Catalog # HIV104
Manufactured by
CHEMBIO DIAGNOSTIC SYSTEMS, INC.
3661 HORSEBLOCK RD.
MEDFORD, NY 11763 USA
Tel: 1-800-327-3635
Tel: 1-631-924-1135
Fax: 1-631-924-6033
Email: info@chembio.com
Web Site: www.chembio.com