 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
Recommendations for Preventing Transmission of Infections Among Chronic Hemodialysis PatientsTerms and Abbreviations Used in This Publication
Consultant Meeting to Update Recommendations for the Prevention and Control of Bloodborne and Other Infections Among Chronic Hemodialysis PatientsOctober 5-6, 1999 Atlanta, GeorgiaEXPERT CONSULTANTS
James L. Bailey, M.D. Paul Balter, M.D. Jeffrey Berns, M.D. Evelyn Butera, M.S. Thomas Depner, M.D. Claudia Douglas Evelyn Duncan B.F. Edwards, M.D. Martin S. Favero, Ph.D. John Foreman, M.D. Michael W. Fried, M.D. Vasant Gandhi, M.D. Clifford Glynn Ray Hakim, M.D., Ph.D. Norma Heard J. Michael Lazarus, M.D. Nathan Levin, M.D. Paul Light, M.D. Stan Lindenfield, M.D. Cynthia Marshall Bela T. Matyas, M.D., M.P.H. Joseph Mazilli Barbara McCool, M.S.
Ira Meisels, M.D. Catherine M. Meyers, M.D. Svetlozar Natov, M.D. Cathy D. Nutter Stephen Pastan, M.D. Eileen Peacock, M.S.N. Jacquelyn Polder, M.P.H. Robert Sharbaugh, Ph.D. Gayle Shimokura James Steinberg, M.D. Charlotte Thomas-Hawkins, Ph.D. Stephen Vas, M.D. Brian A.J. Walters, Ph.D. David J. Weber, M.D. Rebecca Wingard, M.S.N. Jay Wish, M.D. AGENCY LIAISON CONSULTANT
Paul W. Eggers, Ph.D.
The following CDC staff members prepared this report: Miriam J. Alter, Ph.D. Jerome I. Tokars, M.D., M.P.H. in consultation with Lawrence Y.C. Agodoa, M.D. Carolyn Y. Neuland, Ph.D. SummaryThese recommendations replace previous recommendations for the prevention of bloodborne virus infections in hemodialysis centers and provide additional recommendations for the prevention of bacterial infections in this setting. The recommendations in this report provide guidelines for a comprehensive infection control program that includes a) infection control practices specifically designed for the hemodialysis setting, including routine serologic testing and immunization; b) surveillance; and c) training and education. Implementation of this program in hemodialysis centers will reduce opportunities for patient-to-patient transmission of infectious agents, directly or indirectly via contaminated devices, equipment and supplies, environmental surfaces, or hands of personnel. Based on available knowledge, these recommendations were developed by CDC after consultation with staff members from other federal agencies and specialists in the field who met in Atlanta on October 5--6, 1999. They are summarized in the Recommendations section. This report is intended to serve as a resource for health-care professionals, public health officials, and organizations involved in the care of patients receiving hemodialysis. INTRODUCTIONThe number of patients with end-stage renal disease treated by maintenance hemodialysis in the United States has increased sharply during the past 30 years. In 1999, more than 3,000 hemodialysis centers had >190,000 chronic hemodialysis patients and >60,000 staff members (1). Chronic hemodialysis patients are at high risk for infection because the process of hemodialysis requires vascular access for prolonged periods. In an environment where multiple patients receive dialysis concurrently, repeated opportunities exist for person-to-person transmission of infectious agents, directly or indirectly via contaminated devices, equipment and supplies, environmental surfaces, or hands of personnel. Furthermore, hemodialysis patients are immunosuppressed (2), which increases their susceptibility to infection, and they require frequent hospitalizations and surgery, which increases their opportunities for exposure to nosocomial infections. Historically, surveillance for infections associated with chronic hemodialysis focused on viral hepatitis, particularly hepatitis B virus (HBV) infection. CDC began conducting national surveillance for hemodialysis-associated hepatitis in 1972 (3,4). Since 1976, this surveillance has been performed in collaboration with the Health Care Financing Administration (HCFA) during its annual facility survey. Other hemodialysisassociated diseases and practices not related to hepatitis have been included over the years (e.g., pyrogenic reactions, dialysis dementia, vascular access infections, reuse practices, vancomycin use), and the system is continually updated to collect data regarding hemodialysisassociated practices and diseases of current interest and importance (5--18). Recommendations for the control of hepatitis B in hemodialysis centers were first published in 1977 (19), and by 1980, their widespread implementation was associated with a sharp reduction in incidence of HBV infection among both patients and staff members (5). In 1982, hepatitis B vaccination was recommended for all susceptible patients and staff members (20). However, outbreaks of both HBV and hepatitis C virus (HCV) infections continue to occur among chronic hemodialysis patients. Epidemiologic investigations have indicated substantial deficiencies in recommended infection control practices, as well as a failure to vaccinate hemodialysis patients against hepatitis B (21,22). These practices apparently are not being fully implemented because staff members a) are not aware of the practices and their importance, b) are confused regarding the differences between standard (i.e., universal) precautions recommended for all health-care settings and the additional precautions necessary in the hemodialysis setting, and c) believe that hepatitis B vaccine is ineffective for preventing HBV infection in chronic hemodialysis patients (22). Bacterial infections, especially those involving vascular access, are the most frequent infectious complication of hemodialysis and a major cause of morbidity and mortality among hemodialysis patients (1). During the 1990s, the prevalence of antimicrobial-resistant bacteria (e.g., methicillin-resistant Staphylococcus aureus [MRSA] and vancomycin-resistant enterococci [VRE]) increased rapidly in health-care settings, including hemodialysis units (18,23). Although numerous outbreaks of bacterial infections in the hemodialysis setting have been reported (24), few studies exist regarding the epidemiology and prevention of endemically occurring bacterial infections in hemodialysis patients, and formal recommendations to prevent such infections have not been published previously. In 1999, CDC initiated a surveillance system for bloodstream and vascular access infections in outpatient hemodialysis centers to determine the frequency of and risk factors for these complications in order to formulate and evaluate strategies for control (25). The recommendations contained in this report were developed by reviewing available data and are based on consultations with specialists in the field. These recommendations provide guidelines for infection control strategies, unique to the hemodialysis setting, that should be used to prevent patient-to-patient transmission of bloodborne viruses and pathogenic bacteria. They are summarized on pages 20--21. These recommendations do not address sources of bacterial and chemical contaminants in dialysis systems, water treatment or distribution, specific procedures for reprocessing dialyzers, clinical practice methods to prevent bacterial infections (e.g., techniques for skin preparation and access), or comprehensive strategies for preventing infections among health-care workers (see Suggested Readings for information on these topics). BACKGROUNDHepatitis B Virus Infection Epidemiology Incidence and Prevalence. In 1974, the incidence of newly acquired (i.e., acute) HBV infection among chronic hemodialysis patients in the United States was 6.2%, and selected hemodialysis centers reported rates as high as 30% (4). By 1980, nationwide incidence among patients had decreased to 1% (5), and by 1999, to 0.06% (18) (CDC, unpublished data, 2001), with only 3.5% of all centers reporting newly acquired infections. Prevalence of chronic HBV infection (i.e., hepatitis B surface antigen [HBsAg] positivity) among hemodialysis patients declined from 7.8% in 1976 to 3.8% in 1980 and to 0.9% by 1999 (5,18) (CDC, unpublished data, 2001). In 1999, a total of 27.7% of 3,483 centers provided dialysis to >1 patient with either acute or chronic HBV infection (CDC, unpublished data, 2001). Transmission. HBV is transmitted by percutaneous (i.e., puncture through the skin) or permucosal (i.e., direct contact with mucous membranes) exposure to infectious blood or to body fluids that contain blood, and the chronically infected person is central to the epidemiology of HBV transmission. All HBsAg-positive persons are infectious, but those who are also positive for hepatitis B e antigen (HBeAg) circulate HBV at high titers in their blood (108--9 virions/mL) (26,27). With virus titers in blood this high, body fluids containing serum or blood also can contain high levels of HBV and are potentially infectious. Furthermore, HBV at titers of 102--3 virions/mL can be present on environmental surfaces in the absence of any visible blood and still result in transmission (28,29). HBV is relatively stable in the environment and remains viable for at least 7 days on environmental surfaces at room temperature (29). HBsAg has been detected in dialysis centers on clamps, scissors, dialysis machine control knobs, and doorknobs (30). Thus, blood-contaminated surfaces that are not routinely cleaned and disinfected represent a reservoir for HBV transmission. Dialysis staff members can transfer virus to patients from contaminated surfaces by their hands or gloves or through use of contaminated equipment and supplies (30). Most HBV infection outbreaks among hemodialysis patients were caused by cross-contamination to patients via a) environmental surfaces, supplies (e.g., hemostats, clamps), or equipment that were not routinely disinfected after each use; b) multiple dose medication vials and intravenous solutions that were not used exclusively for one patient; c) medications for injection that were prepared in areas adjacent to areas where blood samples were handled; and d) staff members who simultaneously cared for both HBV-infected and susceptible patients (21,31--35). Once the factors that promote HBV transmission among hemodialysis patients were identified, recommendations for control were published in 1977 (19). These recommendations included a) serologic surveillance of patients (and staff members) for HBV infection, including monthly testing of all susceptible patients for HBsAg; b) isolation of HBsAg-positive patients in a separate room; c) assignment of staff members to HBsAg-positive patients and not to HBV-susceptible patients during the same shift; d) assignment of dialysis equipment to HBsAg-positive patients that is not shared by HBV-susceptible patients; e) assignment of a supply tray to each patient (regardless of serologic status); f) cleaning and disinfection of nondisposable items (e.g., clamps, scissors) before use on another patient; g) glove use whenever any patient or hemodialysis equipment is touched and glove changes between each patient (and station); and h) routine cleaning and disinfection of equipment and environmental surfaces. The segregation of HBsAg-positive patients and their equipment from HBV-susceptible patients resulted in 70%--80% reductions in incidence of HBV infection among hemodialysis patients (7,36--38). National surveillance data for 1976--1989 indicated that incidence of HBV infection was substantially lower in hemodialysis units that isolated HBsAg-positive patients, compared with those that did not (7,10). The success of isolation practices in preventing transmission of HBV infection is linked to other infection control practices, including routine serological surveillance and routine cleaning and disinfection. Frequent serologic testing for HBsAg detects patients recently infected with HBV quickly so isolation procedures can be implemented before cross-contamination can occur. Environmental control by routine cleaning and disinfection procedures reduces the opportunity for cross-contamination, either directly from environmental surfaces or indirectly by hands of personnel. Despite the current low incidence of HBV infection among hemodialysis patients, outbreaks continue to occur in chronic hemodialysis centers. Investigations of these outbreaks have documented that HBV transmission resulted from failure to use recommended infection control practices, including a) failure to routinely screen patients for HBsAg or routinely review results of testing to identify infected patients; b) assignment of staff members to the simultaneous care of infected and susceptible patients; and c) sharing of supplies, particularly multiple dose medication vials, among patients (21). In addition, few patients had received hepatitis B vaccine (21). National surveillance data have demonstrated that independent risk factors among chronic hemodialysis patients for acquiring HBV infection include the presence of >1 HBV-infected patient in the hemodialysis center who is not isolated, as well as a <50% hepatitis B vaccination rate among patients (15). HBV infection among chronic hemodialysis patients also has been associated with hemodialysis provided in the acute-care setting (21,39). Transmission appeared to stem from chronically infected HBV patients who shared staff members, multiple dose medication vials, and other supplies and equipment with susceptible patients. These episodes were recognized when patients returned to their chronic hemodialysis units, and routine HBsAg testing was resumed. Transmission from HBV-infected chronic hemodialysis patients to patients undergoing hemodialysis for acute renal failure has not been documented, possibly because these patients are dialyzed for short durations and have limited exposure. However, such transmission could go unrecognized because acute renal failure patients are unlikely to be tested for HBV infection. Clinical Features and Natural History HBV causes both acute and chronic hepatitis. The incubation period ranges from 45--160 days (mean: 120 days), and the onset of acute disease is usually insidious. Infants, young children (aged <10 years), and immunosuppressed adults with newly acquired HBV infection are usually asymptomatic (40). When present, clinical symptoms and signs might include anorexia, malaise, nausea, vomiting, abdominal pain, and jaundice. Extrahepatic manifestations of disease (e.g., skin rashes, arthralgias, and arthritis) can also occur (41). The case fatality rate after acute hepatitis B is 0.5%--1%. In adults with normal immune status, most (94%--98%) recover completely from newly acquired HBV infections, eliminating virus from the blood and producing neutralizing antibody that creates immunity from future infection (40,42). In immunosuppressed persons (including hemodialysis patients), infants, and young children, most newly acquired HBV infections result in chronic infection. Although the consequences of acute hepatitis B can be severe, most of the serious sequelae associated with the disease occur in persons in whom chronic infection develops. Although persons with chronic HBV infection are often asymptomatic, chronic liver disease develops in two-thirds of these persons, and approximately 15%--25% die prematurely from cirrhosis or liver cancer (43--45). Subtypes of HBV exist, and infection or immunization with one subtype confers immunity to all subtypes. However, reinfection or reactivation of latent HBV infection has been reported among certain groups of immunosuppressed patients, including those who have undergone renal transplant and those infected with human immunodeficiency virus (HIV) (46,47). These patients were positive for antibody to hepatitis B core antigen (anti-HBc), with or without antibody to HBsAg (anti-HBs), and subsequently developed detectable levels of HBsAg. The frequency with which this occurs is unknown. Monotherapy with alpha interferon or lamivudine is approved by the U.S. Food and Drug Administration (FDA) to treat patients with chronic hepatitis B (48,49). Although the dosage of lamivudine should be modified based on creatinine clearance in patients with renal impairment, no additional dose modification is necessary after routine hemodialysis. The emergence of lamivudine-resistant variants has caused concern regarding long-term use of this drug. Screening and Diagnostic Tests Serologic Assays. Several well-defined antigen-antibody systems are associated with HBV infection, including HBsAg and anti-HBs; hepatitis B core antigen (HBcAg) and anti-HBc; and HBeAg and antibody to HBeAg (anti-HBe). Serologic assays are commercially available for all of these except HBcAg because no free HBcAg circulates in blood. One or more of these serologic markers are present during different phases of HBV infection (Table 1) (42). The presence of HBsAg is indicative of ongoing HBV infection and potential infectiousness. In newly infected persons, HBsAg is present in serum 30--60 days after exposure to HBV and persists for variable periods. Transient HBsAg positivity (lasting <18 days) can be detected in some patients during vaccination (50,51). Anti-HBc develops in all HBV infections, appearing at onset of symptoms or liver test abnormalities in acute HBV infection, rising rapidly to high levels, and persisting for life. Acute or recently acquired infection can be distinguished by presence of the immunoglobulin M (IgM) class of anti-HBc, which persists for approximately 6 months. In persons who recover from HBV infection, HBsAg is eliminated from the blood, usually in 2--3 months, and anti-HBs develops during convalescence. The presence of anti-HBs indicates immunity from HBV infection. After recovery from natural infection, most persons will be positive for both anti-HBs and anti-HBc, whereas only anti-HBs develops in persons who are successfully vaccinated against hepatitis B. Persons who do not recover from HBV infection and become chronically infected remain positive for HBsAg (and anti-HBc), although a small proportion (0.3% per year) eventually clear HBsAg and might develop anti-HBs (45). In some persons, the only HBV serologic marker detected is anti-HBc (i.e., isolated anti-HBc). Among most asymptomatic persons in the United States tested for HBV infection, an average of 2% (range: <0.1%--6%) test positive for isolated anti-HBc (52); among injecting-drug users, however, the rate is 24% (53). In general, the frequency of isolated anti-HBc is directly related to the frequency of previous HBV infection in the population and can have several explanations. This pattern can occur after HBV infection among persons who have recovered but whose anti-HBs levels have waned or among persons who failed to develop anti-HBs. Persons in the latter category include those who circulate HBsAg at levels not detectable by current commercial assays. However, HBV DNA has been detected in <10% of persons with isolated anti-HBc, and these persons are unlikely to be infectious to others except under unusual circumstances involving direct percutaneous exposure to large quantities of blood (e.g., transfusion) (54). In most persons with isolated anti-HBc, the result appears to be a false positive. Data from several studies have demonstrated that a primary anti-HBs response develops in most of these persons after a three-dose series of hepatitis B vaccine (55,56). No data exist on response to vaccination among hemodialysis patients with this serologic pattern. A third antigen, HBeAg, can be detected in serum of persons with acute or chronic HBV infection. The presence of HBeAg correlates with viral replication and high levels of virus (i.e., high infectivity). Anti-HBe correlates with the loss of replicating virus and with lower levels of virus. However, all HBsAg-positive persons should be considered potentially infectious, regardless of their HBeAg or anti-HBe status. Nucleic Acid Detection. HBV infection can be detected using qualitative or quantitative tests for HBV DNA. These tests are not FDA-approved and are most commonly used for patients being managed with antiviral therapy (49,57). Hepatitis B Vaccine Hepatitis B vaccine has been recommended for both hemodialysis patients and staff members since the vaccine became available in 1982 (20). By 1999, a total of 55% of patients and 88% of staff members had been vaccinated (18) (CDC, unpublished data, 2001). Two types of vaccine have been licensed and used in the United States: plasma-derived and recombinant. Plasma-derived vaccine is no longer available in the United States, but is produced in several countries and used in many immunization programs worldwide. Recombinant vaccines available in the United States are Recombivax HB™ (Merck & Company, Inc., West Point, Pennsylvania) and Engerix-B® (SmithKline Beecham Biologicals, Philadelphia, Pennsylvania). Recombivax HB™ contains 10--40 µg of HBsAg protein per mL, whereas Engerix-B® contains 20 µg/mL. Primary vaccination comprises three intramuscular doses of vaccine, with the second and third doses given 1 and 6 months, respectively, after the first. An alternative schedule of four doses given at 0, 1, 2, and 12 months to persons with normal immune status or at 0, 1, 2, and 6 months to hemodialysis patients has been approved for Engerix-B®. Immunogenicity. The recommended primary series of hepatitis B vaccine induces a protective anti-HBs response (defined as >10 milli-International Units [mIU]/mL) in 90%--95% of adults with normal immune status. The major determinant of vaccine response is age, with the proportion of persons developing a protective antibody response declining to 84% among adults aged >40 years and to 75% by age 60 years (58,59). Other host factors that contribute to decreased immunogenicity include smoking, obesity, and immune suppression. Compared with adults with normal immune status, the proportion of hemodialysis patients who develop a protective antibody response after vaccination (with higher dosages) is lower. For those who receive the three-dose schedule, the median is 64% (range: 34%--88%) (60--65), and for those who receive the four-dose schedule, the median is 86% (range: 40%--98%) (66--72). Limited data indicate that concurrent infection with HCV does not interfere with development of protective levels of antibody after vaccination, although lower titers of anti-HBs have been reported after vaccination of HCV-positive patients compared with HCV-negative patients (65,73--75). Some studies have demonstrated that higher antibody response rates could be achieved by vaccinating patients with chronic renal failure before they become dialysis dependent, particularly patients with mild or moderate renal failure. After vaccination with four 20 µg doses of recombinant vaccine, a protective antibody response developed in 86% of predialysis adult patients with serum creatinine levels <4.0 mg/dl (mean: 2.0 mg/dl) compared with 37% of those with serum creatinine levels >4.0 mg/dl (mean: 9.5 mg/dl), only 12% of whom were predialysis patients (76). In an earlier study, a lower response to recombinant vaccine among predialysis patients was reported, possibly because patients with more severe renal failure were included (77,78). Although no data exist on the response of pediatric hemodialysis patients to vaccination with standard pediatric doses, 75%--97% of those who received higher dosages (20 µg) on either the three- or four-dose schedule developed protective levels of anti-HBs (79--81). In the one study that evaluated vaccine response among children with chronic renal failure before they became dialysis dependent, high response rates were achieved after four-20 µg doses in both predialysis and dialysis-dependent patients, although predialysis patients had higher peak antibody titers (82). Vaccine Efficacy. For persons with normal immune status, controlled clinical trials have demonstrated that protection from acute and chronic HBV infection is virtually complete among those who develop a protective antibody response after vaccination (83,84). Among hemodialysis patients, controlled clinical trials conducted in other countries demonstrated efficacy of 53%--78% after preexposure immunization (85,86). However, no efficacy was demonstrated in the one trial performed in the United States (62). When the latter trial was designed, the sample size was calculated based on an annual incidence rate among susceptible patients of 13.8% (i.e., the rate observed during 1976--1979, the period before the start of the trial). However, by the time the trial was conducted, the incidence rate had declined by >60%, and the sample size was inadequate for detecting a difference in infection rates between vaccinated and placebo groups. Although efficacy was not demonstrated in this study, no infections occurred among persons who developed and maintained protective levels of anti-HBs. Furthermore, since the hepatitis B vaccine became available, no HBV infections have been reported among vaccinated hemodialysis patients who maintained protective levels of anti-HBs. This observation has been particularly striking during HBV infection outbreaks in this setting (21). In addition, a case-control study indicated that the risk for HBV infection was 70% lower among hemodialysis patients who had been vaccinated (87). Thus, most hemodialysis patients can be protected from hepatitis B by vaccination, and maintaining immunity among these patients reduces the frequency and costs of serologic screening (88). Revaccination of Nonresponders. Among persons who do not respond to the primary three-dose series of hepatitis B vaccine, 25%--50% of those with normal immune status respond to one additional vaccine dose, and 50%--75% respond to three additional doses (59,84). A revaccination regimen that includes serologic testing after one or two additional doses of vaccine appears to be no more cost-effective than serologic testing performed after all three additional doses (89). For persons found to be nonresponders after six doses of vaccine, no data exist to indicate that additional doses would induce an antibody response. Few studies have been conducted of the effect of revaccination among hemodialysis patients who do not respond to the primary vaccine series. Response rates to revaccination varied from 40%--50% after two or three additional 40 µg intramuscular doses to 64% after four additional 10 µg intramuscular doses (69,70,90--94). Antibody Persistence. Among adults with normal immune status who responded to a primary vaccine series with a protective antibody level, antibody remained above protective levels in 40%--87% of persons after 9--15 years (95--98). Only short-term data are available for hemodialysis patients. Among adults who responded to the primary vaccination series, antibody remained detectable for 6 months in 80%--100% (median: 100%) of persons and for 12 months in 58%--100% (median: 70%) (61,64--69,71,85,99--103). Among successfully immunized hemodialysis patients whose antibody titers subsequently declined below protective levels, limited data indicate that virtually all respond to a booster dose (75). Duration of Vaccine-Induced Immunity. Among persons with normal immune status who respond to the primary series of hepatitis B vaccine, protection against hepatitis B persists even when antibody titers become undetectable (97). However, among hemodialysis patients who respond to the vaccine, protection against hepatitis B is not maintained when antibody titers fall below protective levels. In the U.S. vaccine efficacy trial, three hemodialysis patients who responded to the primary vaccination series developed HBV infection (62). One had received a kidney transplant 6 months before onset of infection, and anti-HBs had declined to borderline protective levels in the other two persons. In all three patients, infection resolved. Alternative Routes of Administration. Among adults with normal immune status, intradermal administration of low doses of hepatitis B vaccine results in lower seroconversion rates (55%--81%) (104--106), and no data exist on long-term protection from this route of administration. Among infants and children, intradermal vaccination results in poor immunogenicity. Data are insufficient to evaluate alternative routes (e.g., intradermal) for vaccination among hemodialysis patients. Hepatitis C Virus Infection Epidemiology Incidence and Prevalence. Data are limited on incidence of HCV infection among chronic hemodialysis patients. During 1982--1997, the incidence of non-A, non-B hepatitis among patients reported to CDC's national surveillance system decreased from 1.7% to 0.2% (18). The validity of these rates is uncertain because of inherent difficulties in diagnosing non-A, non-B hepatitis and probable variability in the application of diagnostic criteria by different dialysis centers. However, the downward trend can partially be explained by a decline in the rate of transfusion-associated disease after 1985 (107,108). Since 1990, limited data from U.S. studies using testing for antibody to HCV (anti-HCV) to evaluate the incidence of HCV infection have reported annual rates of 0.73%--3% among hemodialysis patients (109,110). None of the patients who seroconverted had received transfusions in the interim or were injecting-drug users. During 1992--1999, national surveillance data indicated that the proportion of centers that tested patients for anti-HCV increased from 22% to 56% (18) (CDC, unpublished data, 2001). In 1999, nationwide prevalence of anti-HCV was 8.9%, with some centers reporting prevalences >40% (CDC, unpublished data, 2001). Other studies of hemodialysis patients in the United States have reported anti-HCV prevalences of 10%--36% among adults (109,111,112) and 18.5% among children (113). Transmission. HCV is most efficiently transmitted by direct percutaneous exposure to infectious blood, and like HBV, the chronically infected person is central to the epidemiology of HCV transmission. Risk factors associated with HCV infection among hemodialysis patients include history of blood transfusions, the volume of blood transfused, and years on dialysis (114). The number of years on dialysis is the major risk factor independently associated with higher rates of HCV infection. As the time patients spent on dialysis increased, their prevalence of HCV infection increased from an average of 12% for patients receiving dialysis <5 years to an average of 37% for patients receiving dialysis >5 years (109,112,115). These studies, as well as investigations of dialysis-associated outbreaks of hepatitis C, indicate that HCV transmission most likely occurs because of inadequate infection control practices. During 1999--2000, CDC investigated three outbreaks of HCV infection among patients in chronic hemodialysis centers (CDC, unpublished data, 1999 and 2000). In two of the outbreaks, multiple transmissions of HCV occurred during periods of 16--24 months (attack rates: 6.6%--17.5%), and seroconversions were associated with receiving dialysis immediately after a chronically infected patient. Multiple opportunities for cross-contamination among patients were observed, including a) equipment and supplies that were not disinfected between patient use; b) use of common medication carts to prepare and distribute medications at patients' stations; c) sharing of multiple dose medication vials, which were placed at patients' stations on top of hemodialysis machines; d) contaminated priming buckets that were not routinely changed or cleaned and disinfected between patients; e) machine surfaces that were not routinely cleaned and disinfected between patients; and f) blood spills that were not cleaned up promptly. In the third outbreak, multiple new infections clustered at one point in time (attack rate: 27%), suggesting a common exposure event. Although the specific results of this investigation are pending, multiple opportunities for cross-contamination from chronically infected patients also were observed in this unit. In particular, supply carts were moved from one station to another and contained both clean supplies and blood-contaminated items, including small biohazard containers, sharps disposal boxes, and used vacutainers containing patients' blood. Clinical Features and Natural History HCV causes both acute and chronic hepatitis. The incubation period ranges from 14--180 days (average: 6--7 weeks) (116). Persons with newly acquired (acute) HCV infection typically are either asymptomatic or have a mild clinical illness. The course of acute hepatitis C is variable, although elevations in serum alanine aminotransferase (ALT) levels, often in a fluctuating pattern, are the most characteristic feature. Fulminant hepatic failure after acute hepatitis C is rare. Most (average: 94%) hemodialysis patients with newly acquired HCV infection have elevated serum ALT levels (117--121). Elevations in serum ALT levels often precede anti-HCV seroconversion. Among prospectively followed transfusion recipients who developed acute HCV infection, elevated ALT levels preceded anti-HCV seroconversion (as measured by second generation assays) in 59%, and anti-HCV was detectable in most patients (78%) within 5 weeks after their first ALT elevation (122). However, elevations in ALT or aspartate aminotransferase (AST) levels can occur that are not related to viral hepatitis, and compared with ALT, AST is a less specific indicator of HCV-related liver disease among hemodialysis patients. In a recent outbreak investigation, only 28% of 25 hemodialysis patients with newly observed elevations in AST levels tested anti-HCV positive (CDC, unpublished data, 1999). After acute HCV infection, 15%--25% of persons with normal immune status appear to resolve their infection without sequelae as defined by sustained absence of HCV RNA in serum and normalization of ALT (123). In some persons, ALT levels normalize, suggesting full recovery, but this is frequently followed by ALT elevations that indicate progression to chronic disease. Chronic HCV infection develops in most infected persons (75%--85%). Of persons with chronic HCV infection, 60%--70% have persistent or fluctuating ALT elevations, indicating active liver disease (123). Although similar rates of chronic liver disease have been observed among HCV-infected chronic hemodialysis patients (based on liver biopsy results), these patients might be less likely to have biochemical evidence of active liver disease (124). In seroprevalence studies of chronic hemodialysis patients, ALT elevations were reported in a median of 33.9% (range: 6%--73%) of patients who tested positive for anti-HCV (117,124--136). No clinical or epidemiologic features among patients with acute infection have been reported to be predictive of either persistent infection or chronic liver disease. Most studies have reported that cirrhosis develops in 10%--20% of persons who have had chronic hepatitis C for 20--30 years, and hepatocellular carcinoma in 1%--5% (123). Extrahepatic manifestations of chronic HCV infection are considered to be of immunologic origin and include cryoglobulinemia, membranoproliferative glomerulonephritis, and porphyria cutanea tarda (137). At least six different genotypes and >90 subtypes of HCV exist, with genotype 1 being the most common in the United States (138,139). Unlike HBV, infection with one HCV genotype or subtype does not protect against reinfection or superinfection with other HCV strains (139). Alpha interferon alone or in combination with ribavirin is FDA-approved for the treatment of chronic hepatitis C (48,140,141). Combination therapy should be used with caution in patients with creatinine clearance <50 mL/minute and generally is contraindicated in patients with renal failure (141,142). Interferon monotherapy results in low sustained virologic response rates (141,142). Screening and Diagnostic Tests Serologic Assays. The only FDA-approved tests for diagnosis of HCV infection are those that measure anti-HCV and include enzyme immunoassays (EIAs) and a supplemental recombinant immunoblot assay (RIBA™) (116). These tests detect anti-HCV in >97% of infected persons, but do not distinguish between acute, chronic, or resolved infection. The average time from exposure to seroconversion is 8--9 weeks (122). Anti-HCV can be detected in 80% of patients within 15 weeks after exposure, in >90% within 5 months, and in >97% within 6 months (122,143). In rare instances, seroconversion can be delayed until 9 months after exposure (143,144). Anti-HCV persists indefinitely in most persons, but does not protect against reinfection. As with any screening test, the positive predictive value of EIAs for anti-HCV is directly related to the prevalence of infection in the population and is low in populations with an HCV-infection prevalence <10% (145,146). Supplemental testing with a more specific assay (i.e., RIBA™) of a specimen with a positive anti-HCV result by EIA prevents reporting of false-positive results, particularly in settings where asymptomatic persons are being tested. Results of seroprevalence studies among chronic hemodialysis patients have indicated that 57%--100% of EIA positive results were RIBA™ positive (124,126,128,133,135,147--152), and 53%--100% were HCV RNA positive by reverse transcriptase polymerase chain reaction (RT-PCR) testing (117,127,129,134,135). Nucleic Acid Detection. The diagnosis of HCV infection also can be made by qualitatively detecting HCV RNA using gene amplification techniques (e.g., RT-PCR) (116). HCV RNA can be detected in serum or plasma within 1--2 weeks after exposure and weeks before onset of ALT elevations or the appearance of anti-HCV. In rare instances, detection of HCV RNA might be the only evidence of HCV infection. Although a median of 3.4% (range: 0%--28%) of chronic hemodialysis patients who tested anti-HCV negative were HCV RNA positive, this might be an overestimate because follow-up samples to detect possible antibody seroconversions were not obtained on these patients (117,118,126--128,130,131,133,134,148--154). Although not FDA-approved, RT-PCR assays for HCV infection are used commonly in clinical practice and are commercially available. Most RT-PCR assays have a lower limit of detection of 100--1,000 viral genome copies per mL. With adequate optimization of RT-PCR assays, 75%--85% of persons who are positive for anti-HCV and >95% of persons with acute or chronic hepatitis C will test positive for HCV RNA. Some HCV-infected persons might be only intermittently HCV RNA positive, particularly those with acute hepatitis C or with end-stage liver disease caused by hepatitis C. To minimize false-negative results, blood samples collected for RT-PCR should not contain heparin, and serum must be separated from cellular components within 2--4 hours after collection and preferably stored frozen at -20 C or -70 C (155). If shipping is required, frozen samples should be protected from thawing. Because of assay variability, rigorous quality assurance and control should be in place in clinical laboratories performing this assay, and proficiency testing is recommended. Quantitative assays for measuring the concentration (i.e., titer) of HCV RNA have been developed and are available from commercial laboratories (156). These assays also are not FDA-approved and are less sensitive than qualitative RT-PCR assays (157). Quantitative assays should not be used as a primary test to confirm or exclude the diagnosis of HCV infection or to monitor the endpoint of treatment, and sequential measurement of HCV RNA levels has not proven useful in managing patients with hepatitis C. Other Bloodborne Viruses Hepatitis Delta Virus Infection Delta hepatitis is caused by the hepatitis delta virus (HDV), a defective virus that causes infection only in persons with active HBV infection. The prevalence of HDV infection is low in the United States, with rates of <1% among HBsAg-positive persons in the general population and >10% among HBsAg-positive persons with repeated percutaneous exposures (e.g., injecting-drug users, persons with hemophilia) (158). Areas of the world with high endemic rates of HDV infection include southern Italy, parts of Africa, and the Amazon Basin. Few data exist on the prevalence of HDV infection among chronic hemodialysis patients, and only one transmission of HDV between such patients has been reported in the United States (159). In this episode, transmission occurred from a patient who was chronically infected with HBV and HDV to an HBsAg-positive patient after a massive bleeding incident; both patients received dialysis at the same station. HDV infection occurs either as a co-infection with HBV or as a superinfection in a person with chronic HBV infection. Co-infection usually resolves, but superinfection frequently results in chronic HDV infection and severe disease. High mortality rates are associated with both types of infection. A serologic test that measures total antibody to HDV (anti-HDV) is commercially available. Human Immunodeficiency Virus Infection During 1985--1999, the percentage of U.S. hemodialysis centers that reported providing chronic hemodialysis for patients with HIV infection increased from 11% to 39%, and the proportion of hemodialysis patients with known HIV infection increased from 0.3% to 1.4% (18) (CDC, unpublished data, 2001). HIV is transmitted by blood and other body fluids that contain blood. No patient-to-patient transmission of HIV has been reported in U.S. hemodialysis centers. However, such transmission has been reported in other countries; in one case, HIV transmission was attributed to mixing of reused access needles and inadequate disinfection of equipment (160). HIV infection is usually diagnosed with assays that measure antibody to HIV, and a repeatedly positive EIA test should be confirmed by Western blot or another confirmatory test. Antiretroviral therapies for HIV-infected hemodialysis patients are commonly used and appear to be improving survival rates among this population. However, hepatotoxicity associated with certain protease inhibitors might limit the use of these drugs, especially in patients with underlying liver dysfunction (161). Bacterial Infections Epidemiology Disease Burden. The annual mortality rate among hemodialysis patients is 23%, and infections are the second most common cause, accounting for 15% of deaths (1). Septicemia (10.9% of all deaths) is the most common infectious cause of mortality. In various studies evaluating rates of bacterial infections in hemodialysis outpatients, bacteremia occurred in 0.63%--1.7% of patients per month and vascular access infections (with or without bacteremia) in 1.3%--7.2% of patients per month (162--170). National surveillance data indicated that 4%--5% of patients received intravenous vancomycin during a 1-month period (and additional patients received other antimicrobials) (18). Although data on vancomycin use can be used to derive an estimate of the prevalence of suspected infections, the proportion of patients receiving antimicrobials who would fit a formal case definition for bacterial infection is unknown. Infection Sites. In a study of 27 French hemodialysis centers, 28% of 230 infections in hemodialysis patients involved the vascular access, whereas 25% involved the lung, 23% the urinary tract, 9% the skin and soft tissues, and 15% other or unknown sites (165). Thirty-three percent of infections involved either the vascular access site or were bacteremias of unknown origin, many of which might have been caused by occult access infections. Thus, the vascular access site was the most common site for infection, but accounted for only one-third of infections. However, access site infections are particularly important because they can cause disseminated bacteremia or loss of the vascular access. Vascular Access Infections. Vascular access infections are caused (in descending order of frequency) by S. aureus, coagulase-negative staphylococci (CNS), gram-negative bacilli, nonstaphylococcal gram-positive cocci (including enterococci), and fungi (171). The proportion of infections caused by CNS is higher among patients dialyzed through catheters than among patients dialyzed through fistulas or grafts. The primary risk factor for access infection is access type, with catheters having the highest risk for infection, grafts intermediate, and native arteriovenous (AV) fistulas the lowest (168). Other potential risk factors for vascular access infections include a) location of the access in the lower extremity; b) recent access surgery; c) trauma, hematoma, dermatitis, or scratching over the access site; d) poor patient hygiene; e) poor needle insertion technique; f) older age; g) diabetes; h) immunosuppression; and i) iron overload (164,167,172--175). Transmission. Bacterial pathogens causing infection can be either exogenous (i.e, acquired from contaminated dialysis fluids or equipment) or endogenous (i.e., caused by invasion of bacteria present in or on the patient). Exogenous pathogens have caused numerous outbreaks, most of which resulted from inadequate dialyzer reprocessing procedures (e.g., contaminated water or inadequate disinfectant) or inadequate treatment of municipal water for use in dialysis. During 1995--1997, four outbreaks were traced to contamination of the waste drain port on one type of dialysis machine (176). Recommendations to prevent such outbreaks are published elsewhere (171). Contaminated medication vials also are a potential source of bacterial infection for patients. In 1999, an outbreak of Serratia liquefaciens bloodstream infections and pyrogenic reactions among hemodialysis patients was traced to contamination of vials of erythropoietin. These vials, which were intended for single use, were contaminated by repeated puncture to obtain additional doses and by pooling of residual medication into a common vial (177). Endogenous pathogens first colonize the patient and later cause infection. Colonization means that microorganisms have become resident in or on the body (e.g., in the nares or stool); a culture from the site is positive, but no symptoms or signs of infection exist. Colonization with potentially pathogenic microorganisms, often unknown to staff members, is common in patients with frequent exposure to hospitals and other health-care settings. Colonization most often occurs when microorganisms are transmitted from a colonized or infected source patient to another patient on the hands of health-care workers who do not comply with infection control precautions. Less commonly, contamination of environmental surfaces (e.g., bed rails, countertops) plays a role (178). Infection occurs when microorganisms invade the body, damaging tissue and causing signs or symptoms of infection, and is aided by invasive devices (e.g., the hemodialysis vascular access). Evidence exists that when prevalence of colonization in a population is less frequent, infection in that population will also be less frequent, and infection control recommendations for hemodialysis units are designed to prevent colonization (179). Additional measures designed to prevent infection from colonizing organisms (e.g., using aseptic technique during vascular access) are presented elsewhere (180). Antimicrobial Resistance Antimicrobial-resistant bacteria are more common in patients with severe illness, who often have had multiple hospitalizations or surgical procedures, and in those who have received prolonged courses of antimicrobial agents. In health-care settings, including hemodialysis centers, such patients can serve as a source for transmission. Clinically important drug-resistant bacteria that commonly cause health-care--associated infections include MRSA, methicillin-resistant CNS, VRE, and multidrug-resistant gram negative rods, including strains of Pseudomonas aeruginosa, Stenotrophomonas maltophilia, and Acinetobacter species, some of which are resistant to all available antimicrobials. In addition, strains of S. aureus with intermediate resistance to vancomycin and other glycopeptide antibiotics have recently been reported; these strains are called vancomycin-intermediate S. aureus (VISA) or glycopeptide-intermediate S. aureus (GISA) (181,182). Intermediate resistance to vancomycin is reported even more frequently among CNS (183,184). Hemodialysis patients have played a prominent role in the epidemic of vancomycin resistance. In 1988, a renal unit in London, England, reported one of the first cases of VRE (185). In three studies, 12%--22% of hospitalized patients infected or colonized with VRE were receiving hemodialysis (178,186,187). Furthermore, three of the first five patients identified with VISA (or GISA) were on chronic hemodialysis, and one had received acute dialysis (182). Prevalence of VRE has increased rapidly at U.S. hospitals; among intensive care unit patients with nosocomial infections reported to the National Nosocomial Infections Surveillance (NNIS) system, the percentage of enterococcal isolates resistant to vancomycin increased from 0.5% in 1989 to 25.2% in 1999 (23) (CDC, unpublished data, 2000). This increase is attributable to patient-to-patient transmission in health-care settings and transmission of resistant genes among previously susceptible enterococci. Once vancomycin resistance has been transferred to a patient, antimicrobials select for resistant organisms, causing them to increase in number relative to susceptible organisms. Prevalence of VRE colonization among patients varies in different health-care settings; in hemodialysis centers, the reported prevalence in stool samples ranged from 1% to 9% (188,189). In one center with a prevalence of 9%, three patients developed VRE infections in 1 year (188). Vancomycin Use Dialysis patients have played a prominent role in the epidemic of vancomycin resistance because this drug is used commonly in these patients, in part because vancomycin can be conveniently administered to patients when they come in for hemodialysis treatments. However, two studies indicate that cefazolin, a first-generation cephalosporin, could be substituted for vancomycin in many patients (190,191). One of these studies reported that many pathogens causing infections in hemodialysis patients are susceptible to cefazolin (190), and both studies reported therapeutic cefazolin blood levels 48--72 hours after dosing, making in-center administration three times a week after dialysis feasible. Equipment, Supplies, and Environmental Surfaces The hemodialysis machine and its components also can be vehicles for patient-to-patient transmission of bloodborne viruses and pathogenic bacteria (24,192). The external surfaces of the machine are the most likely sources for contamination. These include not only frequently touched surfaces (e.g., the control panel), but also attached waste containers used during the priming of the dialyzers, blood tubing draped or clipped to waste containers, and items placed on tops of machines for convenience (e.g., dialyzer caps and medication vials). Sterilization, Disinfection, and Cleaning A sterilization procedure kills all microorganisms, including highly resistant bacterial spores (24). Sterilization procedures are most commonly accomplished by steam or ethylene oxide gas. For products that are heat sensitive, an FDA-cleared liquid chemical sterilant can be used with a long exposure time (i.e., 3--10 hours). High-level disinfection kills all viruses and bacteria, but not high numbers of bacterial spores. High-level disinfection can be accomplished by heat pasteurization or, more commonly, by an FDA-cleared chemical sterilant, with an exposure time of 12--45 minutes. Sterilants and high-level disinfectants are designed to be used on medical devices, not environmental surfaces. Intermediate-level disinfection kills bacteria and most viruses and is accomplished by using a tuberculocidal "hospital disinfectant" (a term used by the U.S. Environmental Protection Agency [EPA] in registering germicides) or a 1:100 dilution of bleach (300--600 mg/L free chlorine). Low-level disinfection kills most bacteria and is accomplished by using general purpose disinfectants. Intermediate and low-level disinfectants are designed to be used on environmental surfaces; they also can be used on noncritical medical devices, depending on the design and labeling claim. Cleaning eliminates dirt and some bacteria and viruses and is accomplished by using a detergent or detergent germicide. Antiseptics (e.g., formulations with povidone-iodine, hexachlorophene, or chlorhexidene) are designed for use on skin and tissue and should not be used on medical equipment or environmental surfaces. Regardless of the procedure used, cleaning with a germicidal detergent before disinfection (or sterilization) is essential to remove organic material (e.g., blood, mucous, or feces), dirt, or debris. The presence of such material protects microorganisms from the sterilization or disinfection process by physically blocking or inactivating the disinfectant or sterilant. The choice of what procedure or which chemical germicide to use for medical devices, instruments, and environmental surfaces depends on several factors, including the need to maintain the structural integrity and function of the item and how the item will be used. Three general categories of use for medical items are recognized, each of which require different levels of sterilization or disinfection (193). These categories are a) critical, which includes items introduced directly into the bloodstream or normally sterile areas of the body (e.g., needles, catheters, hemodialyzers, blood tubing); b) semicritical, which includes equipment that comes in contact with intact mucous membranes (e.g., fiberoptic endoscopes, glass thermometers); and c) noncritical, which includes equipment that touches only intact skin (e.g., blood pressure cuffs). Semicritical items are not generally used in dialysis units. Internal Pathways of Hemodialysis Machines. In single-pass hemodialysis machines, the internal fluid pathways are not subject to contamination with blood. If a dialyzer leak occurs, dialysis fluid might become contaminated with blood, but this contaminated fluid is discarded through a drain and does not return to the dialysis machine to contaminate predialyzer surfaces. For dialysis machines that use a dialysate recirculating system (e.g., some ultrafiltration control machines and those that regenerate the dialysate), a blood leak in a dialyzer could contaminate the internal pathways of the machine, which could in turn contaminate the dialysis fluid of subsequent patients (192). However, procedures normally practiced after each use (i.e., draining the dialysis fluid and rinsing and disinfecting the machine) will reduce the level of contamination to below infectious levels. In addition, an intact dialyzer membrane will not allow passage of bacteria or viruses (24). Pressure transducer filter protectors are used primarily to prevent contamination and preserve the functioning of the pressure monitoring (i.e., arterial, venous, or both) components of the hemodialysis machine. Hemodialysis machines usually have both external (typically supplied with the blood tubing set) and internal protectors, with the internal protector serving as a backup in case the external transducer protector fails. Failure to use an external protector or to replace the protector when it becomes contaminated (i.e., wetted with saline or blood) can result in contamination of the internal transducer protector, which in turn could allow transmission of bloodborne pathogens (24). However, no epidemiologic evidence exists that contamination of the internal transducer protector caused by failure of the external transducer protector has led to either mixing of blood or the transmission of bloodborne agents. Dialyzer Reprocessing. Approximately 80% of U.S. chronic hemodialysis centers reprocess (i.e., reuse) dialyzers for the same patient (18), and guidelines for reprocessing have been published elsewhere (see Suggested Readings). Although outbreaks of bacterial infections and pyrogenic reactions have occurred because of inadequate reprocessing procedures and failure to maintain standards for water quality, reuse has not been associated with transmission of bloodborne viruses. Any theoretical risk for HBV transmission from reuse of dialyzers would primarily affect staff members who handle these dialyzers. Although no increase in HBV (or HCV) infection among staff members who work in such centers has been reported, many centers do not reuse dialyzers from HBsAg-positive patients (24). Infection Control Precautions for Outpatient Hemodialysis Settings Compared with Inpatient Hospital Settings Contact transmission is the most important route by which pathogens are transmitted in health-care settings, including hemodialysis units. Contact transmission occurs most commonly when microorganisms from a patient are transferred to the hands of a health-care worker who does not comply with infection control precautions, then touches another patient. Less commonly, environmental surfaces (e.g., bed rails, countertops) become contaminated and serve as an intermediate reservoir for pathogens; transmission can occur when a worker touches the surface then touches a patient or when a patient touches the surface. In the hemodialysis setting, contact transmission plays a major role in transmission of bloodborne pathogens. If a health-care worker's hands become contaminated with virus-infected blood from one patient, the worker can transfer the virus to a second patient's skin or blood line access port, and the virus can be inoculated into that patient when the skin or access port is punctured with a needle. Contact transmission can be prevented by hand hygiene (i.e., hand washing or use of a waterless hand rub), glove use, and disinfection of environmental surfaces. Of these, hand hygiene is the most important. In addition, nonsterile disposable gloves provide a protective barrier for workers' hands, preventing them from becoming soiled or contaminated, and reduce the likelihood that microorganisms present on the hands of personnel will be transmitted to patients. However, even with glove use, hand washing is needed because pathogens deposited on the outer surface of gloves can be detected on hands after glove removal, possibly because of holes or defects in the gloves, leakage at the wrist, or contamination of hands during glove removal (194). Standard Precautions are the system of infection control precautions recommended for the inpatient hospital setting (195). Standard Precautions are used on all patients and include use of gloves, gown, or mask whenever needed to prevent contact of the health-care worker with blood, secretions, excretions, or contaminated items. In addition to Standard Precautions, more stringent precautions are recommended for hemodialysis units because of the increased potential for contamination with blood and pathogenic microorganisms (see Infection Control Practices Recommended for Hemodialysis Units). For example, infection control practices for hemodialysis units restrict the use of common supplies, instruments, medications, and medication trays and prohibit the use of a common medication cart. For certain patients, including those infected or colonized with MRSA or VRE, contact precautions are used in the inpatient hospital setting. Contact precautions include a) placing the patient in a single room or with another patient infected or colonized with the same organism; b) using gloves whenever entering the patient's room; and c) using a gown when entering the patient's room if the potential exists for the worker's clothing to have substantial contact with the patient, environmental surfaces, or items in the patient's room. Workers also should wear a gown if the patient has diarrhea, an ileostomy, a colostomy, or wound drainage not contained by a dressing. However, contact precautions are not recommended in hemodialysis units for patients infected or colonized with pathogenic bacteria for several reasons. First, although contact transmission of pathogenic bacteria is well-documented in hospitals, similar transmission has not been well-documented in hemodialysis centers. Transmission might not be apparent in dialysis centers, possibly because it occurs less frequently than in acute-care hospitals or results in undetected colonization rather than overt infection. Also, because dialysis patients are frequently hospitalized, determining whether transmission occurred in the inpatient or outpatient setting is difficult. Second, contamination of the patient's skin, bedclothes, and environmental surfaces with pathogenic bacteria is likely to be more common in hospital settings (where patients spend 24 hours a day) than in outpatient hemodialysis centers (where patients spend approximately 10 hours a week). Third, the routine use of infection control practices recommended for hemodialysis units, which are more stringent than the Standard Precautions routinely used in hospitals, should prevent transmission by the contact route. RECOMMENDATIONSRationale Preventing transmission among chronic hemodialysis patients of bloodborne viruses and pathogenic bacteria from both recognized and unrecognized sources of infection requires implementation of a comprehensive infection control program. The components of such a program include infection control practices specifically designed for the hemodialysis setting, including routine serologic testing and immunization, surveillance, and training and education (Box). The infection control practices recommended for hemodialysis units will reduce opportunities for patient-to-patient transmission of infectious agents, directly or indirectly via contaminated devices, equipment and supplies, environmental surfaces, or hands of personnel. These practices should be carried out routinely for all patients in the chronic hemodialysis setting because of the increased potential for blood contamination during hemodialysis and because many patients are colonized or infected with pathogenic bacteria. Such practices include additional measures to prevent HBV transmission because of the high titer of HBV and its ability to survive on environmental surfaces. For patients at increased risk for transmission of pathogenic bacteria, including antimicrobial-resistant strains, additional precautions also might be necessary in some circumstances. Furthermore, surveillance for infections and other adverse events is required to monitor the effectiveness of infection control practices, as well as training and education of both staff members and patients to ensure that appropriate infection control behaviors and techniques are carried out. Infection Control Practices for Hemodialysis Units In each chronic hemodialysis unit, policies and practices should be reviewed and updated to ensure that infection control practices recommended for hemodialysis units are implemented and rigorously followed (see Recommended Infection Control Practices for Hemodialysis Units at a Glance). Intensive efforts must be made to educate new staff members and reeducate existing staff members regarding these practices. Infection Control Precautions for All Patients During the process of hemodialysis, exposure to blood and potentially contaminated items can be routinely anticipated; thus, gloves are required whenever caring for a patient or touching the patient's equipment. To facilitate glove use, a supply of clean nonsterile gloves and a glove discard container should be placed near each dialysis station. Hands always should be washed after gloves are removed and between patient contacts, as well as after touching blood, body fluids, secretions, excretions, and contaminated items. A sufficient number of sinks with warm water and soap should be available to facilitate hand washing. If hands are not visibly soiled, use of a waterless antiseptic hand rub can be substituted for hand washing. Any item taken to a patient's dialysis station could become contaminated with blood and other body fluids and serve as a vehicle of transmission to other patients either directly or by contamination of the hands of personnel. Therefore, items taken to a patient's dialysis station, including those placed on top of dialysis machines, should either be disposed of, dedicated for use only on a single patient, or cleaned and disinfected before being returned to a common clean area or used for other patients. Unused medications or supplies (e.g., syringes, alcohol swabs) taken to the patient's station should not be returned to a common clean area or used on other patients. Additional measures to prevent contamination of clean or sterile items include a) preparing medications in a room or area separated from the patient treatment area and designated only for medications; b) not handling or storing contaminated (i.e., used) supplies, equipment, blood samples, or biohazard containers in areas where medications and clean (i.e., unused) equipment and supplies are handled; and c) delivering medications separately to each patient. Common carts should not be used within the patient treatment area to prepare or distribute medications. If trays are used to distribute medications, clean them before using for a different patient. Intravenous medication vials labeled for single use, including erythropoetin, should not be punctured more than once (196,197). Once a needle has entered a vial labeled for single use, the sterility of the product can no longer be guaranteed. Residual medication from two or more vials should not be pooled into a single vial. If a common supply cart is used to store clean supplies in the patient treatment area, this cart should remain in a designated area at a sufficient distance from patient stations to avoid contamination with blood. Such carts should not be moved between stations to distribute supplies. Staff members should wear gowns, face shields, eye wear, or masks to protect themselves and prevent soiling of clothing when performing procedures during which spurting or spattering of blood might occur (e.g., during initiation and termination of dialysis, cleaning of dialyzers, and centrifugation of blood). Such protective clothing or gear should be changed if it becomes soiled with blood, body fluids, secretions, or excretions. Staff members should not eat, drink, or smoke in the dialysis treatment area or in the laboratory. However, patients can be served meals or eat food brought from home at their dialysis station. The glasses, dishes, and other utensils should be cleaned in the usual manner; no special care of these items is needed. Cleaning and Disinfection. Establish written protocols for cleaning and disinfecting surfaces and equipment in the dialysis unit, including careful mechanical cleaning before any disinfection process (Table 2). If the manufacturer has provided instructions on sterilization or disinfection of the item, these instructions should be followed. For each chemical sterilant and disinfectant, follow the manufacturer's instructions regarding use, including appropriate dilution and contact time. After each patient treatment, clean environmental surfaces at the dialysis station, including the dialysis bed or chair, countertops, and external surfaces of the dialysis machine, including containers associated with the prime waste. Use any soap, detergent, or detergent germicide. Between uses of medical equipment (e.g., scissors, hemostats, clamps, stethoscopes, blood pressure cuffs), clean and apply a hospital disinfectant (i.e., low-level disinfection); if the item is visibly contaminated with blood, use a tuberculocidal disinfectant (i.e., intermediate-level disinfection). For a blood spill, immediately clean the area with a cloth soaked with a tuberculocidal disinfectant or a 1:100 dilution of household bleach (300--600 mg/L free chlorine) (i.e., intermediate-level disinfection). The staff member doing the cleaning should wear gloves, and the cloth should be placed in a bucket or other leakproof container. After all visible blood is cleaned, use a new cloth or towel to apply disinfectant a second time. Published methods should be used to clean and disinfect the water treatment and distribution system and the internal circuits of the dialysis machine, as well as to reprocess dialyzers for reuse (see Suggested Readings). These methods are designed to control bacterial contamination, but will also eliminate bloodborne viruses. For single-pass machines, perform rinsing and disinfection procedures at the beginning or end of the day. For batch recirculating machines, drain, rinse, and disinfect after each use. Follow the same methods for cleaning and disinfection if a blood leak has occurred, regardless of the type of dialysis machine used. Routine bacteriologic assays of water and dialysis fluids should be performed according to the recommendations of the Association for the Advancement of Medical Instrumentation (see Suggested Readings). Venous pressure transducer protectors should be used to cover pressure monitors and should be changed between patients, not reused. If the external transducer protector becomes wet, replace immediately and inspect the protector. If fluid is visible on the side of the transducer protector that faces the machine, have qualified personnel open the machine after the treatment is completed and check for contamination. This includes inspection for possible blood contamination of the internal pressure tubing set and pressure sensing port. If contamination has occurred, the machine must be taken out of service and disinfected using either 1:100 dilution of bleach (300--600 mg/L free chlorine) or a commercially available, EPA-registered tuberculocidal germicide before reuse. Frequent blood line pressure alarms or frequent adjusting of blood drip chamber levels can be an indicator of this problem. Taken separately, these incidents could be characterized as isolated malfunctions. However, the potential public health significance of the total number of incidents nationwide make it imperative that all incidents of equipment contamination be reported immediately to the FDA (800-FDA-1088). Housekeeping staff members in the dialysis facility should promptly remove soil and potentially infectious waste and maintain an environment that enhances patient care. All disposable items should be placed in bags thick enough to prevent leakage. Wastes generated by the hemodialysis facility might be contaminated with blood and should be considered infectious and handled accordingly. These solid medical wastes should be disposed of properly in an incinerator or sanitary landfill, according to local and state regulations governing medical waste disposal. Hemodialysis in Acute-Care Settings. For patients with acute renal failure who receive hemodialysis in acute-care settings, Standard Precautions as applied in all health-care settings are sufficient to prevent transmission of bloodborne viruses. However, when chronic hemodialysis patients receive maintenance hemodialysis while hospitalized, infection control precautions specifically designed for chronic hemodialysis units (see Recommended Practices at a Glance) should be applied to these patients. If both acute and chronic renal failure patients receive hemodialysis in the same unit, these infection control precautions should be applied to all patients. Regardless of where in the acute-care setting chronic hemodialysis patients receive dialysis, the HBsAg status of all such patients should be ascertained at the time of admission to the hospital, by either a written report from the referring center (including the most recent date testing was performed) or by a serologic test. The HBV serologic status should be prominently placed in patients' hospital records, and all health-care personnel assigned to these patients, as well as the infection control practitioner, should be aware of the patients' serologic status. While hospitalized, HBsAg-positive chronic hemodialysis patients should undergo dialysis in a separate room and use separate machines, equipment, instruments, supplies, and medications designated only for HBsAg-positive patients (see Prevention and Management of HBV Infection). While HBsAg-positive patients are receiving dialysis, staff members who are caring for them should not care for susceptible patients. Routine Serologic Testing Chronic Hemodialysis Patients. Routinely test all chronic hemodialysis patients for HBV and HCV infection (see Recommended Practices at a Glance), promptly review results, and ensure that patients are managed appropriately based on their testing results (see later recommendations for each virus). Communicate test results (positive and negative) to other units or hospitals when patients are transferred for care. Routine testing for HDV or HIV infection for purposes of infection control is not recommended. The HBV serologic status (i.e., HBsAg, total anti-HBc, and anti-HBs) of all patients should be known before admission to the hemodialysis unit. For patients transferred from another unit, test results should be obtained before the patients' transfer. If a patient's HBV serologic status is not known at the time of admission, testing should be completed within 7 days. The hemodialysis unit should ensure that the laboratory performing the testing for anti-HBs can define a 10 mIU/mL concentration to determine protective levels of antibody. Routine HCV testing should include use of both an EIA to test for anti-HCV and supplemental or confirmatory testing with an additional, more specific assay (Figure). Use of RT-PCR for HCV RNA as the primary test for routine screening is not recommended because few HCV infections will be identified in anti-HCV negative patients. However, if ALT levels are persistently abnormal in patients who are anti-HCV negative in the absence of another etiology, testing for HCV RNA should be considered (for proper specimen collection and handling, see Hepatitis C Virus Infection, Screening and Diagnostic Tests). Hemodialysis Staff Members. Previously, testing for HBV infection was recommended for all staff members at the time of employment and for susceptible staff members at routine intervals thereafter (198); however, such testing is no longer considered necessary. The risk for HBV infection among hemodialysis staff members is no greater than that for other health-care workers. Thus, routine testing of staff members is not recommended except when required to document response to hepatitis B vaccination (see Postvaccination Testing and Revaccination of Nonresponders). Routine testing of staff members for HCV, HDV, or HIV infection is not recommended. Hepatitis B Vaccination Vaccine Schedule and Dose. Hepatitis B vaccination is recommended for all susceptible chronic hemodialysis patients and for all staff members (Table 3). Vaccination is recommended for pre--end-stage renal disease patients before they become dialysis dependent and for peritoneal and home dialysis patients because they might require in-center hemodialysis. Hepatitis B vaccine should be administered by the intramuscular route and only in the deltoid muscle for adults and children. Intradermal or subcutaneous administration of hepatitis B vaccine is not recommended. If an adult patient begins the vaccine series with a standard dose before beginning hemodialysis treatment, then moves to hemodialysis treatment before completing the series, complete the series using the higher dose recommended for hemodialysis patients (Table 3). No specific recommendations have been made for higher doses for pediatric hemodialysis patients. If a lower than recommended vaccine dose is administered to either adults or children, the dose should be repeated. If the vaccination series is interrupted after the first dose, the second dose should be administered as soon as possible. For the three-dose primary vaccine series, the second and third doses should be separated by an interval of at least 2 months; if only the third dose is delayed, that dose should be administered when convenient. When hepatitis B vaccine has been administered at the same time as other vaccines, no interference with the antibody response of the other vaccines has been demonstrated. Postvaccination Testing and Revaccination of Nonresponders. Test all vaccinees for anti-HBs 1--2 months after the last primary vaccine dose, to determine their response to the vaccine (adequate response is defined as >10 mIU/mL). Patients and staff members who do not respond to the primary vaccine series should be revaccinated with three additional doses and retested for response. No additional doses of vaccine are warranted for those who do not respond to the second series. Evaluate staff members who do not respond to revaccination to determine if they are HBsAg positive (199). Persons who are HBsAg positive should be counseled accordingly (e.g., need for medical evaluation, vaccination of sexual and household contacts). Primary nonresponders to vaccination who are HBsAg negative should be considered susceptible to HBV infection and counseled regarding precautions to prevent HBV infection and the need to obtain postexposure prophylaxis with hepatitis B immune globulin for any known or probable percutaneous or mucosal exposure to HBsAg-positive blood (199). Follow-Up of Vaccine Responders. Retest patients who respond to the vaccine annually for anti-HBs. If anti-HBs declines to <10 mIU/mL, administer a booster dose of hepatitis B vaccine and continue to retest annually. Retesting immediately after the booster dose is not necessary. For staff members who respond to the vaccine, booster doses of vaccine are not necessary, and periodic serologic testing to monitor antibody concentrations is not recommended (199). Patients with a History of Vaccination. Routine childhood vaccination against hepatitis B has been recommended since 1991 and routine adolescent vaccination since 1995 (89,198). Thus, many persons who develop end-stage renal failure will have a history of vaccination against hepatitis B. These persons should have responded to the vaccine when their immune status was normal, but if their anti-HBs levels are <10 mIU/mL when they begin dialysis, they should be revaccinated with a complete primary series. Prevention and Management of HBV Infection Preventing HBV transmission among chronic hemodialysis patients requires a) infection control precautions recommended for all hemodialysis patients; b) routine serologic testing for markers of HBV infection and prompt review of results; c) isolation of HBsAg-positive patients with dedicated room, machine, other equipment, supplies, and staff members; and d) vaccination. Additional infection control practices are needed because of the potential for environmentally mediated transmission of HBV, rather than internal contamination of dialysis machines. The need for routine follow-up testing, vaccination, or isolation is based on patients' serologic status (Table 1 and Recommended Practices at a Glance). HBV-Susceptible Patients. Vaccinate all susceptible patients (see Hepatitis B Vaccination). Test susceptible patients monthly for HBsAg, including those who a) have not yet received hepatitis B vaccine, b) are in the process of being vaccinated, or c) have not adequately responded to vaccination. Although the incidence of HBV infection is low among chronic hemodialysis patients, preventing transmission depends on timely detection of patients converting from HBsAg negative to HBsAg positive and rapid implementation of isolation procedures before cross-contamination can occur. HBsAg Seroconversions. Report HBsAg-positive seroconversions to the local health department as required by law or regulation. When a seroconversion occurs, review all patients' routine laboratory test results to identify additional cases. Perform additional testing as indicated later in this section. Investigate potential sources for infection to determine if transmission might have occurred within the dialysis unit, including review of newly infected patients' recent medical history (e.g., blood transfusion, hospitalization), history of high-risk behavior (e.g., injecting-drug use, sexual activity), and unit practices and procedures. In patients newly infected with HBV, HBsAg often is the only serologic marker initially detected; repeat HBsAg testing and test for anti-HBc (including IgM anti-HBc) 1--2 months later. Six months later, repeat HBsAg testing and test for anti-HBs to determine clinical outcome and need for counseling, medical evaluation, and vaccination of contacts. Patients who become HBsAg negative are no longer infectious and can be removed from isolation. HBV-Infected Patients. To isolate HBsAg-positive patients, designate a separate room for their treatment and dedicate machines, equipment, instruments, supplies, and medications that will not be used by HBV-susceptible patients. Most importantly, staff members who are caring for HBsAg-positive patients should not care for susceptible patients at the same time, including during the period when dialysis is terminated on one patient and initiated on another. Newly opened units should have isolation rooms for the dialysis of HBsAg-positive patients. For existing units in which a separate room is not possible, HBsAg-positive patients should be separated from HBV-susceptible patients in an area removed from the mainstream of activity and should undergo dialysis on dedicated machines. If a machine that has been used on an HBsAg-positive patient is needed for an HBV-susceptible patient, internal pathways of the machine can be disinfected using conventional protocols and external surfaces cleaned using soap and water or a detergent germicide. Dialyzers should not be reused on HBsAg-positive patients. Because HBV is efficiently transmitted through occupational exposure to blood, reprocessing dialyzers from HBsAg-positive patients might place HBV-susceptible staff members at increased risk for infection. Chronically infected patients (i.e., those who are HBsAg positive, total anti-HBc positive, and IgM anti-HBc negative) are infectious to others and are at risk for chronic liver disease. They should be counseled regarding preventing transmission to others, their household and sexual partners should receive hepatitis B vaccine, and they should be evaluated (by consultation or referral, if appropriate) for the presence or development of chronic liver disease according to current medical practice guidelines. Persons with chronic liver disease should be vaccinated against hepatitis A, if susceptible. Chronically infected patients do not require any routine follow-up testing for purposes of infection control. However, annual testing for HBsAg is reasonable to detect the small percentage of HBV-infected patients who might lose their HBsAg. HBV-Immune Patients. Annual anti-HBs testing of patients who are positive for anti-HBs (>10 mIU/mL) and negative for anti-HBc determines the need for booster doses of vaccine to ensure that protective levels of antibody are maintained. No routine follow-up testing is necessary for patients who are positive for both anti-HBs and anti-HBc. HBV-immune patients can undergo dialysis in the same area as HBsAg-positive patients, or they can serve as a geographic buffer between HBsAg-positive and HBV-susceptible patients. Staff members can be assigned to care for both infected and immune patients on the same shift. Isolated Anti-HBc--Positive Patients. Patients who test positive for isolated anti-HBc (i.e., those who are anti-HBc positive, HBsAg negative, and anti-HBs negative) should be retested on a separate serum sample for total anti-HBc, and if positive, for IgM anti-HBc. The following guidelines should be used for interpretation and follow-up:
Prevention and Management of HCV Infection HCV transmission within the dialysis environment can be prevented by strict adherence to infection control precautions recommended for all hemodialysis patients (see Recommended Practices at a Glance). Although isolation of HCV-infected patients is not recommended, routine testing for ALT and anti-HCV is important for monitoring transmission within centers and ensuring that appropriate precautions are being properly and consistently used. HCV-Negative Patients. Monthly ALT testing will facilitate timely detection of new infections and provide a pattern from which to determine when exposure or infection might have occurred. In the absence of unexplained ALT elevations, testing for anti-HCV every 6 months should be sufficient to monitor the occurrence of new HCV infections. If unexplained ALT elevations are observed in patients who are anti-HCV negative, repeat anti-HCV testing is warranted. If unexplained ALT elevations persist in patients who repeatedly test anti-HCV negative, testing for HCV RNA should be considered. Anti-HCV Seroconversions. Report anti-HCV--positive seroconversions to the local health department as required by law or regulation. When a seroconversion occurs, review all other patients' routine laboratory test results to identify additional cases. Perform additional testing as indicated later in this section. Investigate potential sources for infection to determine if transmission might have occurred within the dialysis unit, including review of newly infected patients' recent medical history (e.g., blood transfusion, hospitalization), history of high-risk behavior (e.g., injecting-drug use, sexual activity), and unit practices and procedures. If >1 patient seroconverts from anti-HCV negative to positive during a 6-month period, more frequent (e.g., every 1--3 months) anti-HCV testing of HCV-negative patients could be warranted for a limited time (e.g., 3--6 months) to detect additional infections. If no additional newly infected patients are identified, resume semiannual testing. If ongoing HCV transmission among patients is identified, implement control measures based on results of investigation of potential sources for transmission and monitor their effectiveness (e.g., perform more frequent anti-HCV testing of HCV-negative patients for 6--12 months before resuming semiannual testing). HCV-Positive Patients. Patients who are anti-HCV positive (or HCV RNA positive) do not have to be isolated from other patients or dialyzed separately on dedicated machines. Furthermore, they can participate in dialyzer reuse programs. Unlike HBV, HCV is not transmitted efficiently through occupational exposures. Thus, reprocessing dialyzers from HCV-positive patients should not place staff members at increased risk for infection. HCV-positive persons should be evaluated (by consultation or referral, if appropriate) for the presence or development of chronic liver disease according to current medical practice guidelines. They also should receive information concerning how they can prevent further harm to their liver and prevent transmitting HCV to others (116,141). Persons with chronic liver disease should be vaccinated against hepatitis A, if susceptible. Prevention and Management of HDV Infection Because of the low prevalence of HDV infection in the United States, routine testing of hemodialysis patients is not necessary or recommended. However, if a patient is known to be infected with HDV, or if evidence exists of transmission of HDV in a dialysis center, screening for delta antibody is warranted. Because HDV depends on an HBV-infected host for replication, prevention of HBV infection will prevent HDV infection in a person susceptible to HBV. Patients who are known to be infected with HDV should be isolated from all other dialysis patients, especially those who are HBsAg-positive. Prevention and Management of HIV Infection Routine testing of hemodialysis patients for HIV infection for infection control purposes is not necessary or recommended. However, patients with risk factors for HIV infection should be tested so that, if infected, they can receive proper medical care and counseling regarding preventing transmission of the virus (201). Infection control precautions recommended for all hemodialysis patients (see Recommended Practices at a Glance) are sufficient to prevent HIV transmission between patients. HIV-infected patients do not have to be isolated from other patients or dialyzed separately on dedicated machines. In addition, they can participate in dialyzer reuse programs. Because HIV is not transmitted efficiently through occupational exposures, reprocessing dialyzers from HIV-positive patients should not place staff members at increased risk for infection. Prevention and Management of Bacterial Infections Follow published guidelines for judicious use of antimicrobials, particularly vancomycin, to reduce selection for antimicrobial-resistant pathogens (202). Infection control precautions recommended for all hemodialysis patients (see Recommended Practices at a Glance) are adequate to prevent transmission for most patients infected or colonized with pathogenic bacteria, including antimicrobial-resistant strains. However, additional infection control precautions should be considered for treatment of patients who might be at increased risk for transmitting pathogenic bacteria. Such patients include those with either a) an infected skin wound with drainage that is not contained by dressings (the drainage does not have to be culture positive for VRE, MRSA, or any specific pathogen) or b) fecal incontinence or diarrhea uncontrolled with personal hygiene measures. For these patients, consider using the following additional precautions: a) staff members treating the patient should wear a separate gown over their usual clothing and remove the gown when finished caring for the patient and b) dialyze the patient at a station with as few adjacent stations as possible (e.g., at the end or corner of the unit). SURVEILLANCE FOR INFECTIONS AND OTHER ADVERSE EVENTSDevelop and maintain a separate centralized record-keeping system (e.g., log book or electronic file) to record the results of patients' vaccination status, serologic testing results for viral hepatitis (including ALT), episodes of bacteremia or loss of the vascular access caused by infection (including date of onset, site of infection, genus and species of the infecting organism, and selected antimicrobial susceptibility results),* and adverse events (e.g., blood leaks and spills, dialysis machine malfunctions). Designate a staff person to promptly review the results of routine testing each time such testing is performed and periodically review recorded episodes of bacteremia or vascular access infections. Specify a procedure for actions required when changes occur in test results or in the frequency of episodes of bacteremias or vascular access loss because of infection. Maintain records for each patient that include the location of the dialysis station and machine number used for each dialysis session and the names of staff members who connect and disconnect the patient to and from a machine. INFECTION CONTROL TRAINING AND EDUCATIONTraining and education is recommended for both staff members and patients (or their family care givers). Training should be appropriate to the cognitive level of the staff member, patient, or family member, and rationales should be provided for appropriate infection control behaviors and techniques to increase compliance. Regulations and recommendations regarding infection control training for health-care workers in general, and dialysis personnel in particular, have been previously published (180,203--205). The following recommendations are intended to highlight and augment the earlier recommendations.
FUTURE DIRECTIONSInfection control strategies that prevent and control HBV infection among hemodialysis patients are well-established. Areas that need additional research include determining the ideal hepatitis B vaccine dosage regimen for pre- and postdialysis pediatric patients and for predialysis adult patients, as well as the optimal timing for follow-up testing and administration of booster doses among vaccine responders. In addition, further studies are needed to clarify the specific factors responsible for transmission of HCV among hemodialysis patients and to evaluate the effect of the current recommendations on prevention and control of HCV infection in this setting. Many areas related to bacterial infections in chronic hemodialysis patients need additional information. Studies are needed on the prevalence and epidemiology of bacterial infections among chronic hemodialysis patients and the patient care practices (e.g., those related to vascular access care and puncture) that would be most useful in preventing bacterial infections. Because of the prominent role of dialysis patients in the epidemic of antimicrobial resistance, researchers need to learn more regarding optimal strategies to ensure judicious use of antimicrobials in these patients. Additional topics for future research include determining the frequency of transmission of pathogenic bacteria in the dialysis unit and whether additional precautions are necessary to prevent such transmission. This document is available on the Internet at <http://www.cdc.gov/hepatitis>. Copies also can be obtained by using the order form at this Internet site or by writing the Hepatitis Branch, Mailstop G37, CDC, Atlanta, GA 30333. References
* Hemodialysis units interested in participating in a formal surveillance system for bacterial infections should consult CDC's Surveillance for Bloodstream and Vascular Access Infections in Outpatient Hemodialysis Centers. More information is available on the Internet at <http://www.cdc.gov/ncidod/hip/Dialysis/DSN_manual.PDF>. Table 1 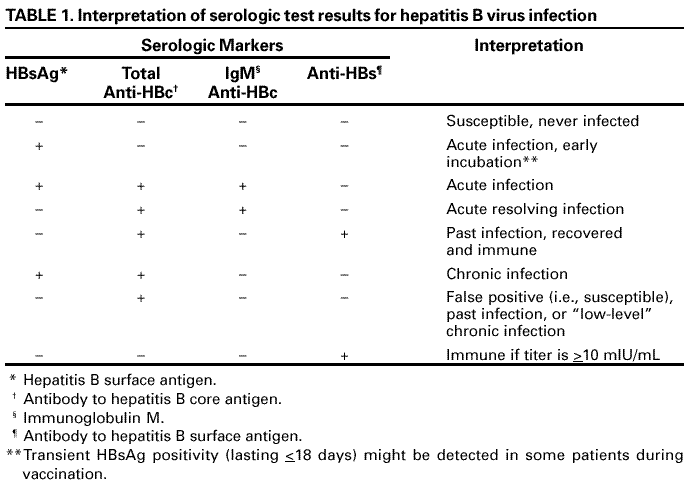 Return to top. Table 2 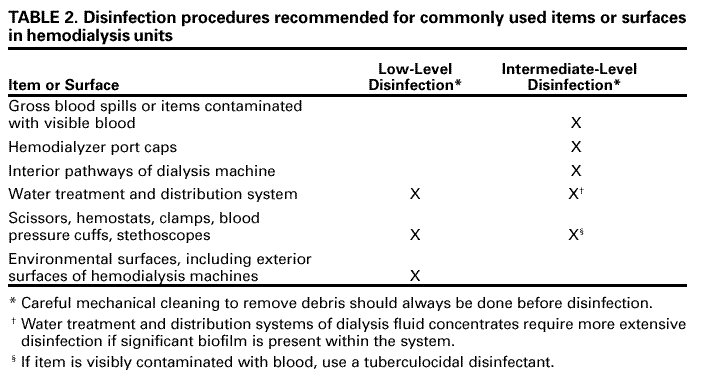 Return to top. Table 3 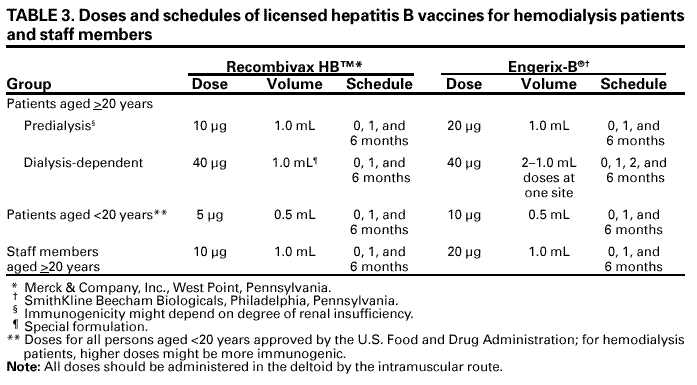 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 4/21/2001 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This page last reviewed 5/1/2001
|