CDER
Report to the Nation: 2002
Table of Contents
Adobe Acrobat version of this document
Communications
Index
Mission
Carry out our mission in consultation with experts in science, medicine and public health and in cooperation with consumers, users, manufacturers, importers, packers, distributors and retailers of human drugs.
Highlights from 2002 include:
- Meeting almost weekly with outside experts on difficult scientific and public health issues.
- Responding to more than 73,000 individual requests for information.
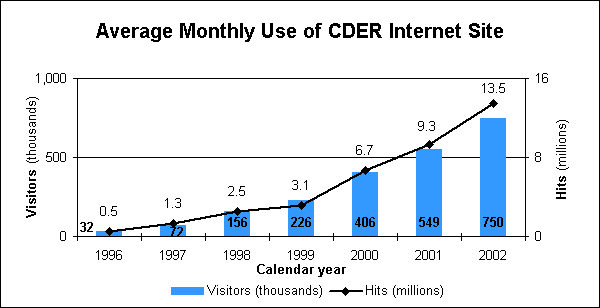
- Receiving nearly 9 million visits and more than 163 million hits on our Internet information site, which has 50,000 pages and documents, five databases and 250,000 hyperlinks.
Stakeholders in drug review, drug quality and safety
We work closely with many organizations on issues of public health and safety, including:
- Consumers, patients and their organizations
- Scientific and professional societies
- Industry and trade associations
- Universities, hospitals and health care professionals
- Federal, state and local government agencies
- Foreign governments
Internet updates
We have 44,000 subscribers to our service that provides daily and weekly e-mail updates of new content on our Web site.
To subscribe, visit http://www.fda.gov/cder/cdernew/listserv.html.
Public participation
We confer with panels of outside experts in science, medicine and public health in meetings open to the public. We assure that patient representatives are included on advisory committees considering medicines for HIV, AIDS, cancer and other serious disorders. We analyze public comments on proposed new rules, and we seek and receive comments on our guidances to industry.
Risk management public hearing. We received valuable input about our ongoing efforts to improve our risk communication and to develop new and effective risk management tools. The purposes of the hearing were to:
- Obtain public input into improving risk management for prescription drugs.
- Identify stakeholders for future collaboration on risk management.
- Improve our understanding of existing risk management tools.
- Guide improvements in and creation of new tools
- Explore assessment strategies.
Workshops
- New approach to plant inspections: Systems inspections. To provide the widest possible industry access, this workshop was held in three locations.
- Pediatric oncology drug development.
We obtained public input on various aspects of developing drugs to treat cancer in children, including prioritization of new and emerging agents, clinical trial design and access to new therapies.
- Scientific workshops.
We held technical workshops to help identify scientific and regulatory issues in such fields as antimicrobial resistance, pharmacogenomics and pharmacogenetics, and drug substance and product specification.
 Back to Index Back to Index
Consumer and industry outreach
- Regulations.
We published seven final regulations, and we sought public comment on another three proposed regulations.
- Guidances.
We published 11 guidances for industry that explain our position on best practices in scientific and technical areas. We published another 19 in draft form seeking public comment.
- Manual of Policies and Procedures.
To foster transparency of our operations, we publish our internal operating policies and procedures on the Internet. We added 23 documents last year.
- Trade press.
We responded to about 2,400 telephone and e-mail requests from the specialized press covering the pharmaceutical industry.
- Exhibits
. We exhibited at 19 conferences, reaching an estimated audience of more than 111,000 consumers, educators and health care professionals.
- Videoconferencing.
We held about 100 domestic and foreign videoconferences for academia, industry and associations.
- CDER Live!
We produced two satellite television broadcasts and Web transmissions for a largely pharmaceutical audience estimated at over 5,000 viewers. The first program dealt with managing the risks of medicines, and the second highlighted new provisions in PDUFA III. Both programs featured our own and industry experts.
- Drug reviews on Internet.
Our Internet site now contains our reviews of more than 200 approved new drugs or new uses for approved drugs.
- Freedom of Information requests.
We responded to nearly 5,000 requests under the Freedom of Information Act.
- General information requests.
We answered more than 32,000 telephone inquiries, 23,000 e-mails and 5,000 letters from consumers, health professionals and industry. We responded to 5,800 requests for documents and guidance publications
Public education campaigns
- Benefits vs. risks of medication use
- Buying drugs from outside the United States
- Buying prescription drugs online
- Drug interactions
- Generic drug quality
- Medical gas safety
- Misuse of prescription pain relievers
- Over-the-counter medicine labels
- Pregnancy and drug use
- Proper drug dosing for children
Many of these are available on the Internet at http://www.fda.gov/cder/consumerinfo/DPAdefaultht.htm.
Ombudsman's activity
In its seventh year, our ombudsman provided informal dispute resolution for both regulated industry and our own employees.
He provided information and guidance to industry, health professionals and consumers.
He helped management identify better ways of conducting business.
Lastly, he represented us in product jurisdiction issues submitted to FDA's Ombudsman's Office. This has been a particularly active area with the development of novel medical products.
 Back to Index Back to Index
We support multiple ways to obtain information about drug products and the laws, regulations and guidances concerning them.
Selected Internet sites
- FDA Internet home page: http://www.fda.gov/
- CDER Internet home page: http://www.fda.gov/cder/
- CDER's consumer drug information sheets for new medicines approved since January 1998:
http://www.fda.gov/cder/consumerinfo/default.htm
- From Test Tube to Patient: New Drug Development in the United States
: http://www.fda.gov/fdac/special/newdrug/ndd_toc.html
- CDER organizational charts: http://www.fda.gov/cder/cderorg.htm
- CDER key officials: http://www.fda.gov/cder/directories/keyoffic.pdf
Telephone
We respond to specific questions about prescription, over-the-counter and generic drugs for human use. You can telephone us toll free at
1-888-INFO FDA or directly at 301-827-4573.
E-mail
We can be contacted at druginfo@cder.fda.gov.
Regular mail
U.S. Food and Drug Administration
Center for Drug Evaluation and Research
Drug Information Division
HFD-240, Room 12B-05
5600 Fishers Lane
Rockville, MD 20857
 Back to Index Back to Index
 Back to Contents Back to Contents
 Back to Top Back to Top  Back to About CDER Back to About CDER
FDA/Center for Drug Evaluation and Research
Last Updated: June 21, 2005
Originator: OTCOM/DCM
HTML by JO, PKS |

