CDER
Report to the Nation: 1999
Table of Contents
Mission
Carry out our mission in consultation with experts in science, medicine and public
health and in cooperation with consumers, users, manufacturers, importers, packers,
distributors and retailers of human drugs.
Stakeholders in drug development and review
We work closely with many organizations during the drug development and review process:
- Industry and trade associations
Consumers and consumer groups
- Universities, hospitals and health care professionals
- Patients, families, care-givers and patient groups
- Federal, state and local government agencies
- Foreign governments
Public Participation
We participated in the FDA program of public meetings with our stakeholders. Top issues
that emerged included risk management, drug safety, direct-to-consumer advertising and of
our processes. We received valuable input from consumer groups, professional societies,
industry and trade association on these issues. A number of groups expressed a willingness
to partner with us in meeting our objectives, especially in the area of providing
information to consumers and health care professionals. Many of these issues will involve
us in an on-going dialogue with our stakeholders as we seek consensus on directions and
priorities.
We confer with panels of outside experts about difficult scientific issues. These
advisory committees met almost weekly last year, and we assure that patient
representatives are included on committees considering medicines for HIV, AIDS, cancer and
other serious disorders. In addition to analyzing required public comments on proposed new
rules, we sought and received comments on our nonbinding guidances to industry.
Consumer and Industry Outreach Efforts
We use a number of modern communication methods to reach our stakeholders including
making information available on the Internet; leveraging with consumer and patient groups
to publish brochures; using videoconferencing and satellite television broadcasting;
disseminating information about new and existing medicines; and making public service
announcements available to print and broadcast media. Highlights of these activities
include:
- Warning consumers about the dangers of products and dietary supplements that contain the
party drug GHB and related substances. We issued warning flyers and posters to more than
2,500 health and fitness organizations, health care associations, amateur and professional
sports organizations and health care publications.
- Surveying and auditing the pharmaceutical industry to assess their readiness for the
year 2000 transition. To help Americans prepare for YK2, we developed an education
campaign designed to alleviate the concerns of consumers, health care professionals and
other special populations about drug product shortages. The campaign discussed the steps
drug manufacturers had taken to assure a sufficient supply of drug products to meet
demand. Messages were channeled through radio, newspapers, magazines, brochures and FDA's
Website.
- Responding to more than 1,250 telephone and e-mail requests from specialized media that
concentrates on the pharmaceutical industry. This was a 25 percent increase from the
previous year.
- Completing successful showings our exhibit and information program at 13 national health
care conferences and meetings, nearly double last year's number.
- Conducting about 100 domestic and foreign videoconferences for academia, industry and
associations, about the same as last year.
OTC drug labeling campaign
We developed and executed the first phase of a public service campaign designed to
promote awareness and understanding of the new over-the-counter drug labels. We explained
the format the new labels and their benefits for making informed decisions.
We developed and produced two black-and-white print public service announcements, two
radio PSAs, five live-read radio scripts and an exhibit display. The PSAs were distributed
to more than 200 nationwide publications, more than 10,000 newspapers, 6,000 radio
stations and 1,000 television stations. Results from the campaign indicate:
- The radio PSAs were played more than 25,300 times, reaching a total of more than 135
million listeners and amassing more than $1.3 million worth of free air time.
- The print PSA appeared in more than 1,000 newspapers across the country and in Time,
Women's Day, Family Circle and other national magazines.
Dissemination Activities
We provide the most current information FDA-regulated drug products and our processes,
policies and regulations in a timely and accurate manner.
- We updated From Test Tube to Patient: Improving Health through Human Drugs, a
popular FDA publication for consumers that describes new drug development in the United
States and highlights our consumer protection role. The publication consolidates accurate
and timely data about the drug development and review process in an easy-to-understand
format. The report has been requested by more than 5,000 individuals and is responsible
for a large number of Internet "hits."
- In conjunction with a nonprofit industry information association, we conducted three
two-hour satellite television broadcasts for industry called "CDER Live!" Our
scientific and regulatory experts engaged in a panel discussion about electronic
submissions, user fees, risk management, product quality, drug safety and other topical
issues in drug development and manufacturing. Each show is broadcast to about 50 sites
across the country. About 5,000 industry executives, scientists and managers view each
telecast.
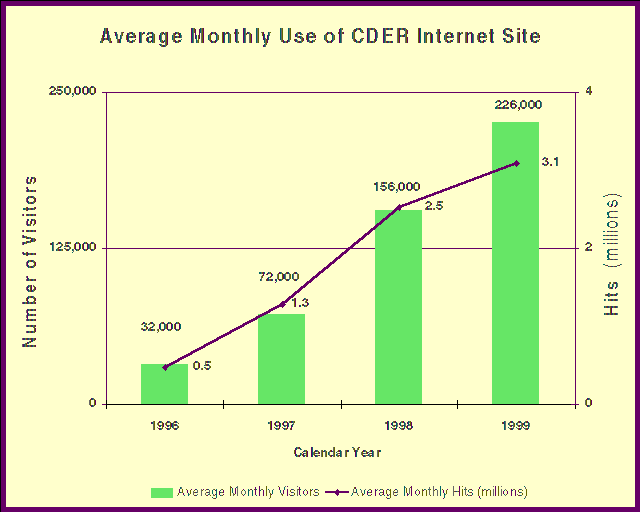
- Our visible presence on the World Wide Web led to an increase in the number of general
information requests by electronic mail from consumers, patients and health care
professionals. We responded to more than 18,200 e-mail requests last year, triple the
number from the previous year.
- In addition, we answered nearly 21,000 telephone inquiries, 4,000 faxes and 2,100
written requests from consumers, pharmacists, doctors, nurses, pharmaceutical and
insurance companies, government agencies and others. Finally, we responded to more than
9,500 requests for documents and guidance publications..
- We developed consumer-friendly, Web-based fact sheets about new molecular entities. The
fact sheets outline the products' labeling, approval dates, uses, possible side-effects,
directions for use, any warnings and other information. In addition, we wrote 33 consumer
information sheets about various drug topics.
Ombudsman's activity
In its fourth year of operations CDER's Ombudsman's Office handled a number of
activities designed to settle situations among employees, the Center, health professionals
and consumers.
The office handled about 80 complaints.
It answered:
- More than 500
e-mails, mostly from consumers and health professionals.
- Approximately 2,000 telephone calls and 20 letters.
In addition, the office held about 50 meetings with external parties.
 Back to
Contents Back to
Contents
Selected Internet sites
Telephone
We respond to specific questions about prescription, over-the-counter and generic drugs
for human use. You can telephone us toll free at 1-888-INFO FDA or directly at
301-827-4573.
E-mail
We can be contacted at DRUGINFO@CDER.FDA.GOV.
Regular mail
U.S. Food and Drug Administration
Center for Drug Evaluation and Research
Drug Information Branch
HFD-210, Room 12B-31
5600 Fishers Lane
Rockville, MD 20857
 Back to Top Back to Top  Back to About CDER Back to About CDER
FDA/Center for Drug Evaluation and Research
Last Updated: March 08, 2001
Originator: OTCOM/DCM
HTML by JO, PKS |

