|
 |

|
 |
 |
|||||
|
|||||||||
|
|
|
|
|
CDER Report to the Nation: 2003 Adobe Acrobat version of this document 1 Drug ReviewIndex
HighlightsMany Americans benefited from last year’s timely reviews of new prescription medicines, over-the-counter medicines and the generic equivalents for both. We approved 21 new medicines that have never been marketed before in this country, known as new molecular entities. We approved 263 generic versions of existing drugs. We authorized three medicines to be sold over the counter without a prescription, and one of them can be used by children. We met or exceeded all 10 performance goals for the fiscal year 2002 receipt cohort, the latest year for which we have full statistics. These are goals we agreed to under legislation authorizing us to collect user fees for drug reviews. In addition to surpassing all goals for original new drug applications, we exceeded both goals for new molecular entities. We conducted 728 foreign and domestic inspections that help protect volunteers for clinical trials from research risks and validate the quality and integrity of data submitted to us. Highlights of new medication options for American consumers include:
Drug approvals for 2003
New Drug ReviewDefinitionsReview and approval times. Review time represents the time that we spend examining the application. Approval time represents our review time plus industry’s response time to our requests for additional information. Median times. Our charts show review and approval times as “medians.” The value for the median time is the number that falls in the middle of the group after the numbers are ranked in order. It provides a truer picture of our performance than average time, which can be unduly influenced by a few very long or short times. Our guide to understanding median approval time statistics is available at http://www.fda.gov/cder/present/MedianAPtime/index.htm. New molecular entities. NMEs contain an active substance that has never before been approved for marketing in any form in the United States. Because of high interest in truly new medicines, we report NMEs separately; however, the charts for NDAs include the NMEs as well. Priority new drugs. These drugs represent significant improvements compared with marketed products. We have a goal of reviewing 90 percent of these applications within six months. Standard new drugs. These drugs have therapeutic qualities similar to those of already marketed products. We have a goal of reviewing 90 percent of these applications within 10 months. Actions and filings. An application is “filed” when we determine it is complete and accept it for review. We make a filing decision within 60 days of receiving an application. Approval is one of the actions that we can take once an application is filed. Other actions include seeking more information from the sponsor. There is no direct connection between applications filed in one year and actions in the same year. Filings provide an idea of what the workload in subsequent years will be. Orphan drugs. We administer a program that provides incentives to develop drugs for use in patient populations of 200,000 or fewer. Sponsors of orphan drugs receive inducements that include seven-year marketing exclusivity, tax credit for the product-associated clinical research, research design assistance from FDA and grants of up to $200,000 per year. Accelerated approval. This program helps make products for serious or life-threatening diseases available earlier in the development process. We base our approval on a promising effect of the drug that can be observed significantly sooner than a long-term clinical benefit. Sponsors perform additional studies to demonstrate long-term clinical benefit. Priority new drugs (N=NME)
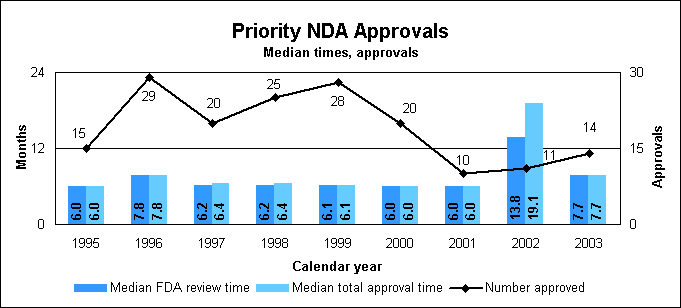
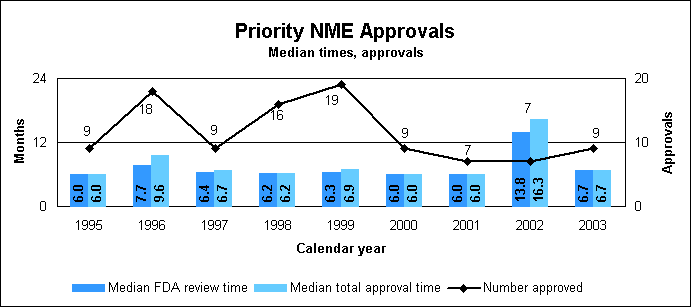
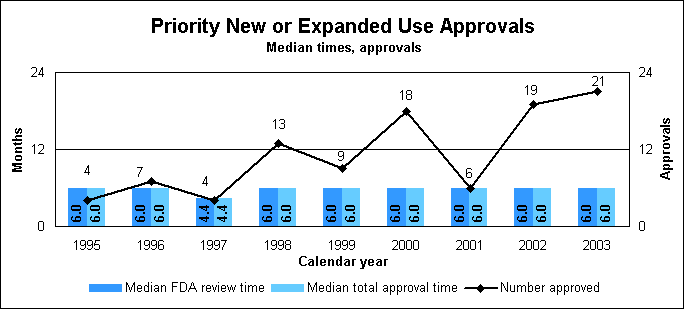
Priority approval, review times down in 2003The median total approval and review times for priority NDAs were 7.7 months each, and the times for priority NMEs were 6.7 months each. The much higher times shown in 2002 were caused by the approval of a number of older applications coupled with a decrease in the number of new applications received. New molecular entities
Orphan drugs (N=NME)
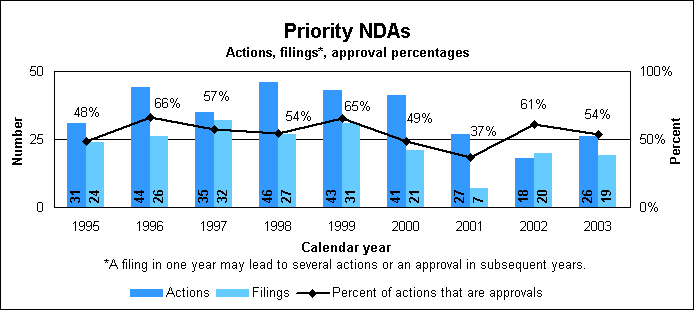
New Drug Review StatisticsPriority new drug statistics
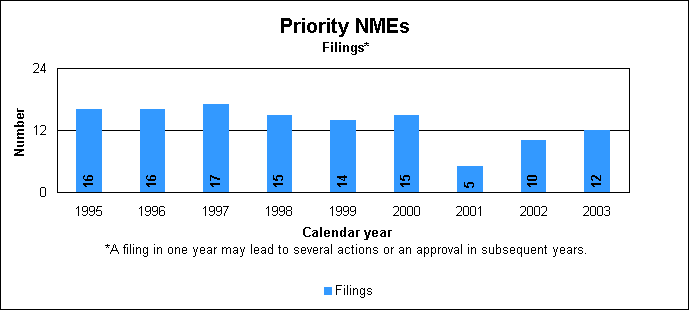
Priority new molecular entity statistics
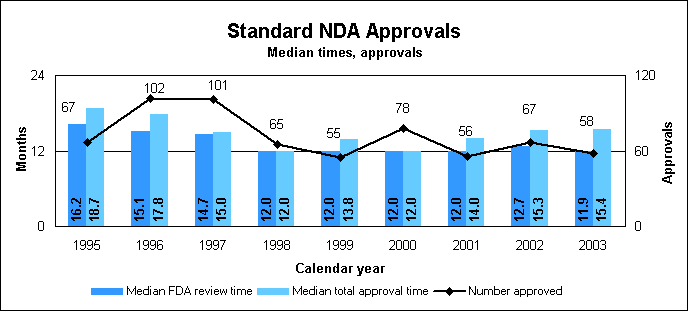
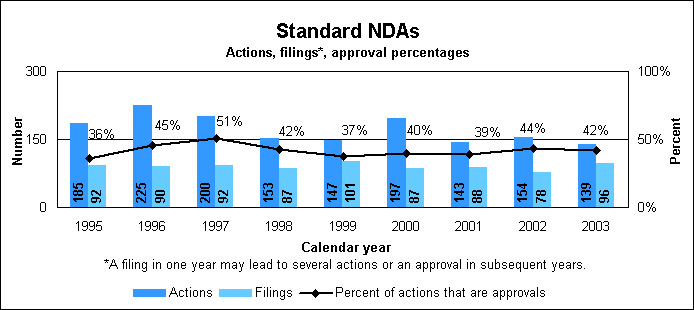
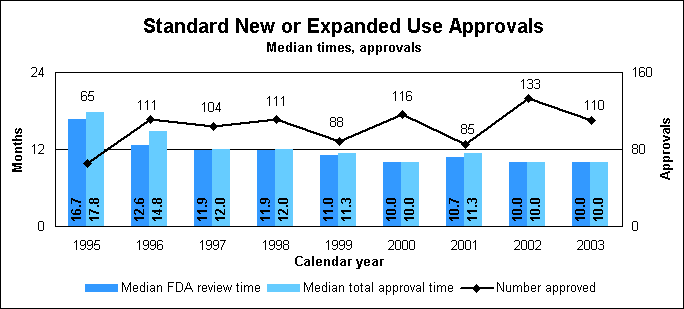
Standard new drug statistics
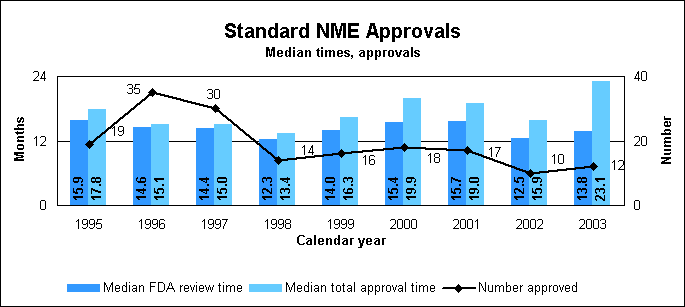
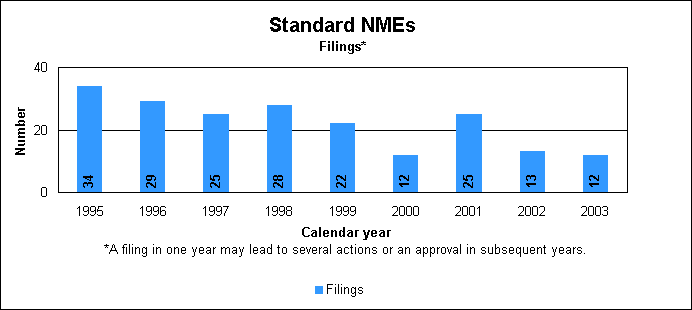
Standard new molecular entity statistics
Notable 2003 new drug approvalsLast year’s approvals benefited people with cancer, HIV infection, heart disease and other disorders. People with cancerAbarelix (Plenaxis) is a palliative treatment for advanced prostate cancer for patients who cannot take other hormone therapies and who have refused surgical castration. Abarelix lowers the male hormone testosterone, which is involved in most prostate cancer growth. A study of 81 men showed they could avoid surgical castration by undergoing at least 12 weeks of treatment with the drug. Some also experienced other benefits, including decreased pain and relief from urinary problems. The drug is marketed under a risk management program because of an increased risk of serious and potentially life-threatening allergic reactions. (NME, P) Aprepitant (Emend) is used in combination with other anti-nausea and anti-vomiting drugs for prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy, including high-dose cisplatin. (NME, P) Gefitinib (Iressa) is a single-agent treatment for patients with advanced non-small cell lung cancer, whose cancer has continued to progress despite treatment with platinum-based chemotherapy and docetaxel. Gefitinib, which received accelerated approval, was developed to block growth stimulatory signals in cancer cells. (NME, P) Imatinib mesylate (Gleevec) received regular approval as a treatment for refractory chronic myeloid leukemia, a rare life threatening form of cancer-affecting about 40,000 people in the United States. The drug was originally approved under the accelerated approval program in 2001. (NDA, P) Palonosetron hydrochloride (Aloxi) is an injectable drug for the prevention of acute nausea and vomiting associated with initial and repeat courses of moderately and highly emetogenic cancer chemotherapy and the prevention of delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy. (NME, S) Bortezomib (Velcade), an orphan drug, is indicated for the treatment of multiple myeloma in patients who have received at least two prior therapies and have demonstrated disease progression on the last therapy. (Orphan, NME, P) Sterile talc powder (Sterile Talc Powder), an orphan drug, is indicated for administering intrapleurally via chest-tube as a sclerosing agent to decrease the recurrence of malignant pleural effusions in symptomatic patients. The pleura is a thin, transparent membrane that covers the lungs and also lines the inside of the chest wall. Normally, only a thin layer of fluid separates the two layers of the pleura. An excessive amount of fluid, called pleural effusion, may accumulate for many reasons, including heart failure, cirrhosis, pneumonia and cancer. (Orphan, NDA, P) People with HIV infectionAtazanavir sulfate (Reyataz) is a protease inhibitor to be used in combination with other anti-retroviral agents for the treatment of adult patients with HIV infection. This drug is the first protease inhibitor that only can be taken once daily. It has a low “pill burden” of two pills each day. Protease inhibitors block the protease enzyme that HIV needs in order to make new viruses. When protease is blocked, HIV makes copies of itself that cannot infect new cells. (NME, P) Emtricitabine (Emtriva) is indicated for the treatment of HIV infection in adults. The drug belongs to the class of anti-HIV agents known as nucleoside reverse transcriptase inhibitors that, when used in combination with other anti-HIV drugs, can block the replication of HIV in a person’s blood. (NME, S) Enfuvirtide (Fuzeon) is an injectable drug indicated for the use in combination with other antiretroviral agents for the treatment of HIV infection in treatment-experienced patients who show evidence of HIV replication despite ongoing antiretroviral therapy. The first member of a new class of medications called fusion inhibitors, the drug received accelerated approval. Enfuvirtide interferes with the entry of HIV into cells by inhibiting fusion of viral and cellular membranes. (NME, P) People with heart diseaseRosuvastatin calcium (Crestor) is an adjunct to diet to lower elevated cholesterol levels, a risk factor for heart disease. It belongs to the class of drugs called HMG-CoA reductase inhibitors, also known as statins. (NME,S) Infectious diseasesDaptomycin (Cubicin) injection treats complicated skin and skin structure infections. These are serious infections, usually occurring in hospitalized patients and include major abscesses, post-surgical skin wound infections and infected ulcers. Daptomycin is the first approved product in a new class of antibiotics called cyclic lipopeptide antibacterial agents. The drug binds to bacterial membranes and rapidly upsets electrical balance. This leads to inhibition of protein, DNA and RNA synthesis, which results in bacterial cell death. (NME, P) Gemifloxacin mesylate (Factive) is indicated for the treatment of community-acquired pneumonia and acute bacterial exacerbation of chronic bronchitis. Gemifloxacin, a synthetic broad-spectrum antibacterial agent for oral administration, is related to the fluoroquinolone class of antibiotics. (NME, S) Sertaconazole nitrate (Ertaczo) cream is indicated for the topical treatment of athlete’s foot (interdigital tinea pedis) in immunocompetent patients 12 years of age and older. Sertaconazole belongs to the imidazole class of antifungals. (NME, S) People with metabolic disordersMiglustat (Zavesca), an orphan drug, is indicated for the treatment of mild to moderate Type I Gaucher disease in adults for whom enzyme replacement therapy is not a therapeutic option due to constraints such as allergy, hypersensitivity or poor venous access. Type 1 Gaucher disease is an inborn error of metabolism that results in disease because of a defect in an enzyme needed to break down the chemical glucocerebroside. The enzyme defect leads to the progressive accumulation of glucocerebroside in the spleen, liver and lymph nodes. Miglustat reduces the body’s formation of glucocerebroside to a level that allows the residual activity of the deficient enzyme to be more effective. (Orphan, NME, S) Pegvisomant (Somavert), an orphan drug, is for the treatment of acromegaly in patients who have an inadequate response to existing therapies or for whom these therapies are not appropriate. Acromegaly is a potentially life-threatening disease caused by an excess of growth hormone. The drug is the first in a new class called growth hormone receptor antagonists. Acromegaly causes changes in facial features and enlarged hands, feet and jaw as well as headaches, profuse sweating, swelling and joint disorders. If untreated, patients with acromegaly often have a shortened life span because of heart and respiratory diseases, diabetes mellitus and cancer. (Orphan, NME, P) People with allergic conjunctivitisEpinastine hydrochloride (ELESTAT) ophthalmic solution is an antihistamine indicated for the prevention of itching associated with allergic conjunctivitis. (NME, S) People with bipolar disorderOlanzapine and fluoxetine hydrochloride (Symbyax) is a combination of two psychotropic agents and is indicated for the treatment of depressive episodes associated with bipolar disorder. Olanzapine belongs to the thienobenzodiazepine class of drugs, and fluoxetine hydrochloride is a selective serotonin reuptake inhibitor. (NDA, P) Older peopleMemantine hydrochloride (Namenda) is the first drug approved for the treatment of patients with moderate to severe Alzheimer’s disease. Previous treatments have been studied in patients with mild to moderate disease. The drug is an N-methyl-D-asparate (NMDA) antagonist and is thought to work by blocking the action of the chemical glutamate. Although memantine hydrochloride helps treat the symptoms of Alzheimer’s disease, there is no evidence that it modifies the underlying pathology of the disease. (NME, S) ChildrenRibavirin (Rebetol) oral solution, an orphan drug, is to be used as part of combination therapy with interferon alpha-2b recombinant (Intron A) for the treatment of chronic hepatitis C among previously untreated pediatric patients at least 3 years of age or older. The drug, a nucleoside analog, was first approved in capsule form in 1998. (Orphan, NDA, P) MenTadalafil (Cialis) and vardenafil (Levitra) are oral medications to treat erectile dysfunction in men. These are the second and third oral products approved for this condition. (Both NME, S) Alfuzosin hydrochloride (Uroxatral) is indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia. The drug is an alpha-blocker and may help to relax the muscles in the prostate and the bladder, which may lessen the symptoms of BPH and improve urine flow. (NME, S) WomenIbandronate sodium (Boniva), indicated for the treatment and prevention of postmenopausal osteoporosis, is a bisphosphonate that inhibits bone resorption. (NME, S) Estradiol (Estrasorb) topical emulsion is an estrogen therapy product in a topical form. The drug treats moderate to severe symptoms of hot flashes and night sweats associated with menopause. Current estrogen products available for treatment include oral pills, transdermal patches and a vaginal ring. (NDA, S) A combination of a progestin (norethindrone) and an estrogen (ethinyl estradiol) (Ovcon 35) is an oral, spearmint-flavored contraceptive tablet that can be chewed or swallowed. This dosage form provides one more alternative to the many types of oral contraceptives currently on the market. (NDA, S) A combination of a progestin (levonorgestrel) and an estrogen (ethinyl estradiol) (Seasonale) provides a new 91-day oral contraceptive regimen. Tablets containing the active hormones are taken for 12 weeks, followed by one week of inactive tablets. Conventional oral contraceptive use is based on a 28-day regimen (21 days of active tablets followed by seven days of inactive tablets). Under this drug’s dosing regimen, the number of expected menstrual periods is reduced from one a month to about one every three months. (NDA, S) Counterterrorism treatmentsInsoluble Prussian blue (Radiogardase), an orphan drug, can be used to treat people exposed to radiation contamination due to harmful levels of cesium-137 or thallium poisoning. The drug works by increasing the rate of elimination of these substances from the body. More information is at http://www.fda.gov/cder/drug/infopage/prussian_blue/. (Orphan, NME, P) Pyridostigmine bromide tablets (Pyridostigmine Bromide) are used as a pretreatment to increase survival after exposure to Soman “nerve gas” poisoning. The product is approved for combat use by U.S. armed forces. This application, sponsored by the U.S. Army, is the first drug approved under the animal efficacy rule. That 2002 regulation allows for use of animal data for evidence of a drug’s effectiveness when the drug cannot be ethically or feasible tested in humans. (NDA, P) Therapeutic biologicsThe review staff and review of some new biologic products were transferred to our center in 2003. This will enhance the efficiency and consistency of reviewing clinically similar products. Medicines transferred include monoclonal antibodies, cytokines, growth factors, enzymes and other therapeutic immunotherapies. This report will begin incorporating statistics on consolidated therapeutic biologic license approvals (BLAs) in 2004. The Center for Biologics Evaluation and Research is reporting these statistics for 2003. During the period Sept.1 to Dec. 31, 2003, when we had official approval authority for therapeutic biologics, we approved one BLA: Efalizumab (Raptiva) is a treatment for adult patients 18 years or older with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy. Drugs@FDADrugs@FDA is a Web site where you can find official information about FDA approved brand name and generic drugs. Use Drugs@FDA to search for:
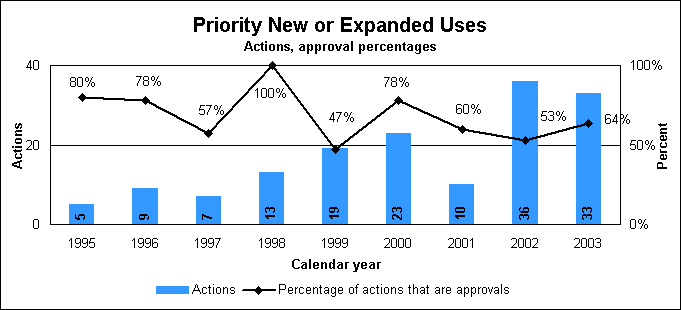
To use Drugs@FDA, go to our home page (http://www.fda.gov/cder) and click on “Drugs@FDA.” Internet resources for drug review statisticsOther drug review statistics are available on our Web site at http://www.fda.gov/cder/rdmt/default.htm. New or Expanded Use ReviewApplications for a new or expanded use, often representing important new treatment options, are formally called “efficacy supplements” to the original new drug application. We have a goal of reviewing standard supplements in 10 months and priority supplements in six months. The new and expanded use review statistics on this page include figures for both priority and standard applications. Statistics on priority new or expanded uses (efficacy supplements)
Priority efficacy supplement reviews
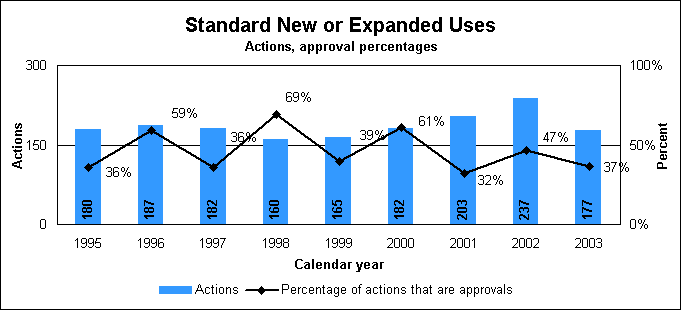
Statistics on standard new or expanded uses (efficacy supplements)
Notable 2003 new or expanded use approvalsCarvedilol (Coreg) is approved for the treatment of patients with left ventricular dysfunction following myocardial infarction. Conjugated estrogens and medroxyprogesterone acetate (Prempro) and conjugated estrogens (Premarin) are available in lower strength doses for the treatment of moderate-to-severe vasomotor symptoms associated with menopause and for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy associated with the menopause. When prescribing solely for the treatment of symptoms of vulvar and vaginal atrophy, topical vaginal products should be considered. Eplerenone (Inspra) tablets improve the survival of congestive heart failure patients who are stablized following an acute heart attack. The drug is the first member of the aldosterone receptor blocker class of drugs to receive approval for this indication. Fondaparinux sodium (Arixtra) injection, used to prevent blood clots, is approved for extended prevention therapy in patients undergoing hip fracture surgery. In a clinical trial that compared 326 patients receiving the drug to 330 patients receiving placebo, blood clots occurred in 1.4 percent of those who received the drug compared to 35 percent of those who received placebo. During the three-week period of treatment, major bleeding rates were 2.4 percent for those administered the drug and 0.6 percent for those administered placebo. Leflunomide (Arava) tablets, a treatment for rheumatoid arthritis, had revised labeling to support the addition of a claim for improved physical function. Losartan potassium (Cozaar) has a new use to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy. The indications section of the label further notes that there is evidence that this benefit does not apply to black patients. Polifeprosan 20 with carmustine implant (Gliadel Wafer) had its indication expanded to include patients with malignant glioma undergoing primary surgical resection. Sirolimus (Rapamune), first approved in 2000 for helping prevent organ rejection in transplant patients, will allow new kidney transplant patients at low to moderate immunologic risk of organ rejection to stop taking cyclosporine two to four months after transplantation. This is the first approval of a cyclosporine-sparing regimen for new kidney transplant patients. Valacyclovir hydrochloride (Valtrex), a treatment for herpes infections first approved in 1995, reduces the risk of heterosexual transmission of genital herpes to susceptible partners with healthy immune systems when used as suppressive therapy in combination with safer sex practices. Orphan approvalsPorfimer sodium (Photofrin), a cancer treatment first approved in 1995, is now approved for the ablation of precancerous lesions in Barrett’s esophagus patients who do not undergo surgery to remove the esophagus. The drug is a photosensitizing agent that can kill cells, including cancer cells, when they are exposed to a particular type of light. A small number of people with Barrett’s esophagus develop pre-cancerous lesions that progress to an often deadly type of cancer of the esophagus called esophageal adenocarcinoma. Somatropin rDNA origin (Zorbtive), a human growth hormone (hGH) produced by recombinant DNA technology, treats short bowel syndrome in patients receiving specialized nutritional support. In human clinical studies, the administration of growth hormone has been shown to enhance the transmucosal transport of water, electrolytes and nutrients. Counterterroism treatmentAtropine (AtroPen) autoinjector is now approved for use in children and adolescents exposed to certain nerve agents or insecticides. The atropine autoinjector, approved since 1973 for use in adults, includes information to support two lower-strength autoinjectors (0.5 mg and 1 mg) and a revised package insert for use in both adult and pediatric populations. Notable pediatric new or expanded usesAtovaquone and proguanil hydrochloride (Malarone Pediatric Tablets) can be used for the treatment of Plasmodium falciparum malaria in pediatric patients weighing 11 pounds to 24.2 pounds. Budesonide (Pulmicort) is approved for use in asthma patients 6 to 12 months; safety information from the study supports the finding that use of the drug in this population may result in systemic effects such as growth suppression. Busulfan (Busulfex) injection, a treatmnent for leukemia, incorporates new pediatric information on dosing, pharmacokinetics and safety. Divalproex (Depakote ER) is now approved for use in pediatric patients for epilepsy. Fentanyl (Duragesic) transdermal system is approved to treat pain in opioid-tolerant pediatric patients 2 years of age and older. The label contains new warning that the drug should only be administered to children if they are opioid-tolerant and 2 years or older. Fluticasone propionate (Flonase and Cutivate). A new study with Flonase nasal spray revealed no significant effect on growth as compared to placebo. Cutivate ointment is only indicated for use in adults; in a pediatric study a lower than normal adrenal function was observed. Fluoxetine hydrochloride (Prozac) was approved to treat children and adolescents 7 to 17 years old for depression (major depressive disorder) and obsessive compulsive disorder. The studies revealed a decrease in both height and weight gain as compared to placebo. Fosinopril sodium (Monopril) incorporates pediatric labeling changes in clinical pharmacology, precautions, adverse reactions, overdosage, and dosage and administration sections of the labeling. Imatinib mesylate (Gleevec) is approved for pediatric patients with Ph+ chronic phase chronic phase chronic myeloid leukemia whose disease has recurred after stem cell transplant or who are resistant to interferon alpha therapy. The clinical benefit for pediatric patients was extrapolated from adult data. Lisinopril (Prinivil and Zestril) is labeled for treatment of hypertension in patients 6 to 16 years of age; information on the preparation of a suspension is provided. Orlistat (Xenical) has revised labeling to provide for use in the management of obesity in adolescent patients aged 12 to 16 years. Priority pediatric labeling changesAn efficacy supplement may change labeling to reflect new information about pediatric use, even if there are no new or expanded uses. Consistent with the mandate in the Best Pharmaceuticals for Children Act, these pediatric supplements received priority reviews last year:
Pediatric Drug Development2003 pediatric drug statistics
The Best Pharmaceuticals for Children Act of 2002 renewed our authority to grant six months of additional marketing exclusivity to manufacturers who conduct and submit pediatric studies in response to our written requests. Last year, we approved 15 pediatric labeling changes as a result of the exclusivity provision. Also, we approved one new molecular entity for use in children (page 18). As of March 31, 2004, we had received 346 proposed pediatric study requests from manufacturers, issued 284 written requests, made 109 exclusivity determinations and added new pediatric information to 71 labels. Approximately one-fourth of the new pediatric labels have important dosing or safety information. Important differences in clearance and metabolism of products are being discovered. This is important because underdosing leads to ineffective treatment and overdosing poses a greater risk of adverse reactions. Pediatric safety signals that have been identified include effects on growth, school behavior and suppression of the adrenal gland. As a result of this pediatric testing we now have eight drugs with new pediatric formulations and six drugs with recipes in their label that provide directions for the pharmacist to compound an age-appropriate formulation. The failure to produce drugs in dosage forms that can be taken by young children such as liquids or chewable tablets can also deny them access to important medications. The BPCA also established a publicly funded contracting process to study drugs that lack patent protection or market exclusivity, referred to as “off-patent.” In consultation with FDA and other pediatric experts, the National Institutes of Health has published three lists of off-patent drugs for which additional pediatric studies are needed. We have issued and forwarded seven written requests for these off-patent drugs to NIH for study through their contracting process. In addition, we have forwarded four written requests for on-patent drugs, which were declined by sponsors, to the Foundation for the National Institutes of Health for study. Conditions with approved pediatric labeling
FDA commissioner’s office oversees pediatric issuesThe Best Pharmaceuticals for Children Act of 2002 mandated the creation of the Office of Pediatric Therapeutics, which began operations in October 2002 in the Commissioner’s office. This office oversees all pediatric issues within FDA including institutional review board referrals to FDA, safety issues, ethical issues and pediatric trial oversight. The office is also responsible for the new Pediatric Advisory Committee that was authorized as part of the Pediatric Research Equity Act. Pediatric Research Equity Act of 2003This law gives FDA the authority to require pediatric studies of new drugs and biologics when such studies are needed to ensure the safe and effective use of these products in children. Internet resourcesOur Web site for up-to-date pediatric labeling changes is at http://www.fda.gov/cder/pediatric/index.htm.
Date created: May 24, 2004
|