 |
 |
 |
 |
FDA Home Page | Search FDA Site | FDA A-Z Index | Contact FDA
MDUFMA requires OCP to provide an annual performance assessment for combination product applications. This section provides performance information for FY 2006 and updates the FY 2005 performance information in the subsection for “Timely and Effective Premarket Review” for reporting the timeliness in days of the reviews of combination products. Unless otherwise noted, all performance information in this section is as of September 30, 2006. Consistent with the mandated functions of the OCP, data highlighted in this section include:
Requirement – Report the Timeliness in Days of the Assignment of Combination Products
FDA is to assign premarket review responsibility for combination products based on the product's PMOA. By submitting an RFD, a company may obtain a formal FDA determination of a combination product’s PMOA and assignment of the lead Center for the product’s premarket review and regulation. OCP must make its jurisdictional determination within 60 days of filing the RFD, or the sponsor’s recommendation of the Center with primary jurisdiction will become the assigned Center.
Requirement |
Requirement |
Request for Designation |
60 calendar days |
Combination Product Assignment Requests |
|
Primary Center |
Number of Product Assignments |
CBER |
7 |
CDER |
5 |
CDRH |
14 |
Pending |
4 |
Total Requested |
30 |
One request for assignment of a combination product was carried over from FY 2005 (pending and not overdue as of October 1, 2005), and 29 requests for combination products were filed during FY 2006 for a total of 30 requests. This reflects a 43 percent increase in the number of RFDs for combination products compared to the 21 RFDs for combination products filed as reported in the FY 2005 OCP Performance Report. Of the 30 FY 2006 requests, 7 combination products were assigned to CBER, 5 to CDER, and 14 to CDRH (see table above). The remaining four requests for combination products were pending and not overdue as of September 30, 2006.
Performance
Of the 26 assignments issued, 19 combination products were determined to be drug-device combinations, 5 were device-biologic combinations, 1 was a drug-biologic combination, and 1 was a drug-device-biologic combination (see table below). All (26 of 26) product assignments were issued within the 60-day time frame, with a median assignment time of 36 days. Assignment time is equal to the number of days from receipt of the RFD to the issuance of the assignment letter.
Combination Product Requests for Assignment |
||||||
Total Requests for Assignment |
Product |
Product |
Product |
Product Assignments |
|
|
30 |
26 |
4 |
0 |
100 |
36 |
3 to 56 |
More detailed FY 2006 RFD performance information, including OCP’s review of RFDs for non-combination products, is available at the OCP Internet site http://www.fda.gov/oc/combination/fy06rfd.html.
Requirement – Report the Number and Types of Combination Products Under Review
FDA is to report the number and types of combination products under review. OCP, CBER, CDER, and CDRH developed a process to collect the necessary data and report on the required information enacted in MDUFMA. This process was implemented April 1, 2003.
The table below reflects the number of original applications for NDAs, BLAs, PMAs, 510(k)s, INDs, IDEs, and HDEs initially classified into one of nine categories of combination products in FY 2006.7 FDA initially categorized 231 original applications under review as combination products.
Number and Types of Combination Products |
|||||||||||
Application Type |
Combination Product Category |
||||||||||
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
TOTALS |
||
Original NDAs |
1 |
11 |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
12 |
|
Original BLAs |
1 |
-- |
1 |
-- |
-- |
-- |
-- |
-- |
-- |
2 |
|
Original PMAs |
-- |
-- |
-- |
2 |
-- |
-- |
-- |
-- |
-- |
2 |
|
Original 510(k)s |
-- |
-- |
-- |
58 |
7 |
-- |
2 |
1 |
5 |
73 |
|
Original INDs |
-- |
59 |
17 |
1 |
8 |
13 |
3 |
17 |
4 |
122 |
|
Original IDEs |
1 |
-- |
-- |
5 |
10 |
-- |
1 |
-- |
1 |
18 |
|
Original HDEs |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
-- |
|
TOTALS |
3 |
70 |
18 |
66 |
26 |
13 |
7 |
18 |
10 |
231 |
|
APPLICATION KEY: |
COMBINATION PRODUCT KEY: 1 = convenience kit or co-package |
||||||||||
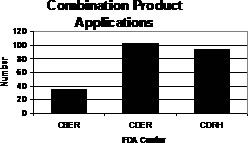
Of the 231 original combination product applications, CBER received and categorized 35 applications as combination products; CDER received and categorized 102 applications as combination products; and CDRH categorized 94 applications as combination products.
FDA is to report the timeliness in days of the reviews of combination products. The table below summarizes the review type and review performance target for original NDAs, BLAs, PMAs, and 510(k)s. PDUFA and MDUFMA established review performance goals for many types of drug, device, and biological product premarket applications.8 These goals reflect current expectations about the portion of premarket applications that will be reviewed within a specified time frame. Performance goals apply to only a portion of all applications of a certain type, and they do not require that every application be reviewed in accordance with the applicable time frame.
User Fee Act |
Original |
Review Type |
Review |
Performance |
|
|---|---|---|---|---|---|
FY |
FY |
||||
Priority |
6 months |
90% |
90% | ||
Standard |
10 months |
90% |
90% | ||
BLAs |
Priority |
6 months |
90% |
90% | |
Standard |
10 months |
90% |
90% | ||
Expedited PMAs |
FDA decision10 |
300 days |
70% |
80% |
|
PMAs |
FDA decision10 |
320 days |
-- |
80% |
|
Original 510(k)s |
“Substantially equivalent” (SE) or “not substantially equivalent” (NSE) decision10 |
90 days |
75% |
75% |
|
BLAs |
Priority |
6 months |
-- |
75% |
|
Standard |
10 months |
-- |
75% |
||
The FDA review performance information for CBER, CDER, and CDRH are based on a fiscal year receipt cohort. This methodology calculates performance information for applications for the fiscal year FDA received them, regardless of when FDA acted on or approved the submissions. This section updates FDA’s review performance on the FY 2005 combination product application submissions and presents FDA’s review performance on the FY 2006 combination product application submissions through September 30, 2006.
FY 2005 Submissions
Fourteen FY 2005 PDUFA submissions identified as CBER-led or CDER-led combination products were reviewed and acted on as of September 30, 2006. These actions included 2 priority and 10 standard NDA combination product submissions and 1 priority and 1 standard BLA combination product submissions (see table below).
PDUFA |
Review |
Review Within |
Reviewed and Acted On11 |
Number on Time12 |
Median or Actual Review Time13 |
Range of Review Time |
|
Min |
Max |
||||||
NDAs |
Priority |
6 months |
2 |
2 |
225 |
182 |
267 |
Standard |
10 months |
10 |
10 |
303 |
293 |
396 |
|
BLAs |
Priority |
6 months |
1 |
1 |
266 |
266 |
266 |
Standard |
10 months |
1 |
1 |
304 |
304 |
304 |
|
FY 2006 Submissions
Two FY 2006 PDUFA submissions identified as CBER-led or CDER-led combination products were reviewed and acted on as of September 30, 2006. These actions included 1 standard NDA and 1 priority BLA combination product submissions (see table below). Additional NDAs and BLAs were under review, with decisions pending. FDA will update the FY 2006 submission data in the FY 2007 OCP Performance Report.
PDUFA |
Review |
Review Within |
Reviewed and Acted On11 |
Number on Time |
Median or Actual Review Time13 |
Range of Review Time |
|
Min |
Max |
||||||
NDAs |
Priority |
6 months |
0 |
-- |
-- |
-- |
-- |
Standard |
10 months |
1 |
1 |
302 |
302 |
302 |
|
BLAs |
Priority |
6 months |
1 |
1 |
183 |
183 |
183 |
Standard |
10 months |
0 |
-- |
-- |
-- |
-- |
|
Performance – CBER-led or CDRH-led Combination Products
FY 2005 Submissions
Fifty-nine FY 2005 MDUFMA submissions identified as CBER-led or CDRH-led combination products had FDA decisions reached as of September 30, 2006. These decisions included 1 expedited PMA, 1 original PMA, and 57 premarket notification [510(k)] combination product submissions (see table below).
MDUFMA Original Application Type14 |
Review |
Review Within |
Decisions Reached11 |
Number on Time 15 |
Median or Actual Review Time16 |
Range of Review Time |
|
Min |
Max |
||||||
Expedited PMAs |
FDA decision |
300 days |
1 |
1 |
290 |
290 |
290 |
PMAs |
FDA decision |
320 days |
1 |
1 |
264 |
264 |
264 |
510(k)s |
SE or NSE decision |
90 days |
57 |
45 |
69 |
16 |
194 |
FY 2006 Submissions
Sixty-six FY 2006 MDUFMA submissions identified as CBER-led or CDRH-led combination products had FDA decisions reached as of September 30, 2006. All decisions made were on 510(k) submissions, which have shorter review times (see table below). Additional PMA and 510(k) combination product submissions were under review, with decisions pending. FDA will update the FY 2006 submissions table in the FY 2007 OCP Performance Report.
MDUFMA Original Application Type14 |
Review |
Review Within |
Decisions Reached11 |
Number on Time 15 |
Median or Actual Review Time16 |
Range of Review Time |
|
Min |
Max |
||||||
Expedited PMAs |
FDA decision |
300 days |
0 |
-- |
-- |
-- |
-- |
PMAs |
FDA decision |
320 days |
0 |
-- |
-- |
-- |
-- |
510(k)s |
SE or NSE decision |
90 days |
66 |
60 |
57 |
10 |
138 |
Requirement – Report the Number of Premarket Reviews of Combination Products That Involved a Consulting Center
FDA is to report the number of premarket reviews of combination products that involved a consulting Center. The table below reflects the Intercenter Requests for Consultative or Collaborative Review forms received and monitored by OCP during FY 2006.17 As the primary assigned Center, CBER requested 40 intercenter consultations (7 consultations with CDER, 33 consultations with CDRH); CDER requested 64 intercenter consultations (2 with CBER and 62 with CDRH); and CDRH requested 231 intercenter consultations (10 with CBER, 221 with CDER).
|
|
Consulting Center |
Number ofConsults |
||
|
|
CBER |
CDER |
CDRH |
|
Primary Assigned Center |
CBER |
-- |
7 |
33 |
40 |
CDER |
2 |
-- |
62 |
64 |
|
CDRH |
10 |
221 |
-- |
231 |
|
|
Totals |
12 |
228 |
95 |
335 |
The monitored Intercenter Requests for Consultative or Collaborative Review forms represent a 22 percent increase over the 275 consults reported in the FY 2005 OCP Performance Report, and are indicative of the growing number of premarket reviews of combination products that involved a consulting Center.
Requirement – Report the Timeliness in Days of Dispute Resolutions Regarding Combination Products
FDA is to report the timeliness in days of dispute resolutions regarding combination products. No formal requests to resolve a dispute regarding the timeliness of a combination product review were received during FY 2006. While this was the fourth straight year no formal requests were received, the “Activities and Impacts for FY 2006, Premarket Review” section of this report provides examples of informal facilitation and resolution of issues related to premarket review. Informal activities help prevent the need for formal dispute resolution.
Table of Contents | Appendix A
![]()