| FDA
> CDRH > CeSub eSubmitter
> Helpful Tips > Navigating through the Tabular Screens
 Navigating Through the Tabular Screens Navigating Through the Tabular Screens
These instructions provide detailed information for using the tabular screens in eSubmitter. To use these instructions, it may be beneficial to have the CeSub eSubmitter application open on your computer.
Tabular Screens:
CeSub eSubmitter allows you to create a table of data by answering certain questions multiple times. Below is a series of examples that will assist in creating a table of data using tabular screens:
A sample tabular screen with no entries made is shown in Figure 1:

Figure 1 : Example of a Tabular Screen with no data
1. Add an entry to a Tabular Screen:
To being creating your table, click on the New button in the top left corner of the screen as depicted in Figure 2 below:

Figure 2: Example of an entry being added to a Tabular Screen
2. Add your data to an entry in a Tabular Screen:
The details tab will appear and the questions are now enabled. Begin entering your data into each question on the screen as depicted in Figure 3 below:
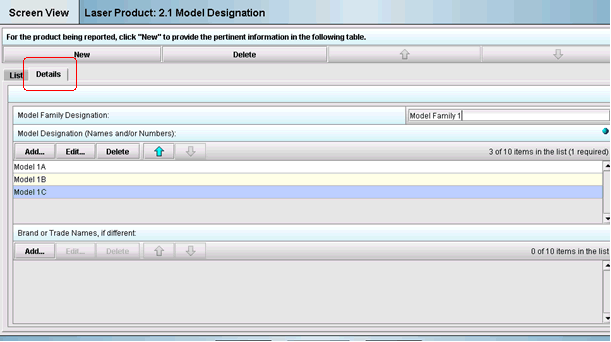
Figure 3: Example of data being entered to create a row
3. View your row entry in the List Tab:
Click on the “List Tab” to view the row entry you have made. You may use the scroll bar at the bottom of the screen to view all entries in the row. See Figure 4 below:
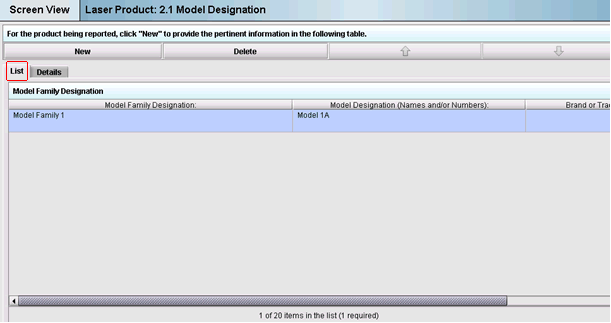
Figure 4: Example of one row of data in the “List Tab”
4. To continue adding data to your table, repeat steps 1, 2, and 3 as necessary:
Figure 5 is an example of a table that consists of three rows and two columns of data. You may rearrange the order of these rows by using the up and down arrows. You may also delete an entry by clicking the “Delete” button.
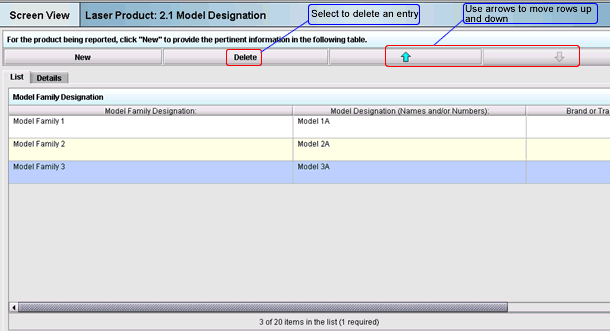
Figure 5: Example of a table of data in a tabular screen being viewed from the “List Tab”
Note: At the bottom of the screen, the number of entries is indicated as “3 of 20 items in the list (1 required)” . This represents the number of entries that may be made (in this case it is 20 entries) and at least one entry is required to complete the submission.
Updated 08-07-2006 |



![]()