 |
 |
 |
 |
FDA Home Page | Search
FDA Site | FDA A-Z Index | Contact
FDA
![]()
Randall W. Lutter, Ph.D.
Acting Deputy Commissioner for Policy
Food and Drug Administration
Subcommittee on Interstate Commerce, Trade, and Tourism
Committee on Commerce, Science, and Transportation
United States Senate
Policy Implications of Importing Drugs into the United States
Mr. Chairman and Members of the Subcommittee, I am Randall W. Lutter, Ph.D., Acting Deputy Commissioner for Policy at the U.S. Food and Drug Administration (FDA or the Agency). Thank you for the opportunity to discuss with you the important issues relating to the importation of prescription drugs.
At FDA, our statutory responsibility is to assure the American public that the drug supply is safe, secure, and reliable. For more than 60 years, the Federal Food, Drug, and Cosmetic (FD&C) Act has helped to ensure that Americans can be confident that when they use an FDA-approved drug, the medicine will be safe and effective and will work as intended in treating their illness and in preventing complications. In carrying out this responsibility, we work, through a variety of steps, to do all we can under the law to make medicines accessible to patients and help doctors and patients use them as effectively as possible. These include: expanding access to essential unapproved treatments that are being studied under FDA investigational new drug applications; approving generic medicines; reducing the time and cost of showing that new medicines are safe and effective; and providing up-to-date information for health professionals and patients to allow them to obtain the benefits and avoid the risks associated with medicines. That is the primary mission of the thousands of dedicated staff, including leading health care experts, doctors, and scientists who work tirelessly at FDA in public service for the American people. FDA remains immensely concerned about unapproved, imported pharmaceuticals whose safety and effectiveness cannot be assured because they originate outside the closed legal structure and regulatory system we are fortunate to have in the United States.
The FD&C Act requires that FDA approve each new drug as safe and effective before marketing. It also authorizes FDA to oversee the production of drugs that are the subject of approved applications, whether manufactured in a facility in the U.S. or a foreign country and imported into the U.S by the manufacturer. By the 1980s, Congress recognized that some foreign entities were importing counterfeit drugs as well as improperly handled and stored drugs into the U.S. For example, at that time, millions of counterfeit birth control pills from Panama found their way into the U.S. drug distribution system. In another case, a counterfeit version of a widely used antibiotic entered the U.S. drug distribution system from a foreign source. These types of activities posed significant risks to American consumers. In 1987, Congress passed the Prescription Drug Marketing Act (PDMA), which strengthened oversight of domestic wholesalers and added the provision to the FD&C Act – 801(d)(1) – that generally prohibits anyone except a drug's manufacturer from reimporting into the U.S. a drug originally manufactured in the U.S. and then sent abroad.
The conclusion of Congress, reflected in current law, is that the safety and effectiveness of imported drugs is best assured by carefully limiting how prescription drugs can be imported into the U.S. as part of a closed drug distribution system. In the case of legally imported drugs, the chain of custody is known for an FDA-approved drug manufactured in an FDA-inspected facility using FDA-approved methods before it is introduced into the U.S. distribution system.
In 2003, Congress tasked the Department of Health and Human Services to examine issues related to drug importation. A task force, chaired by then U.S. Surgeon General Carmona, examined the relevant data, considered testimony from the public and health experts, and then issued the "Report on Prescription Drug Importation" (Task Force Report). This Task Force Report clearly outlines significant safety and economic issues that must be addressed before the widespread importation of unapproved prescription drugs can be permitted. Even though two years have passed since the Task Force Report issued, it is still the most comprehensive examination of the issue and we continue to find evidence confirming its findings.
Some of the key findings identified in the Task Force Report include the following:
Keeping unsafe drugs away from American consumers is an enormous task, as we are faced with a deluge of drugs at points of entry into the U.S. originating from all over the world. We are continually assessing this issue to determine how FDA can best protect American consumers from this threat.
The Internet has created an extraordinary, unregulated marketplace for the sale of unapproved drugs, prescription drugs dispensed without a valid prescription, and products marketed with fraudulent health claims. Patients who buy prescription drugs from a rogue website are at risk of suffering adverse events, some of which can be life threatening. These risks include therapeutic failure due to lack of effect because the drug does not contain the correct dose or active ingredient and potential side effects from inappropriately-prescribed medications, dangerous drug interactions or drug contamination. Patients are also at risk because they often don't know what they are getting when they purchase some of these drugs. Although some patients may receive genuine product, others may unknowingly receive counterfeit copies that contain inert or harmful ingredients, drugs that are expired and have been diverted to illegitimate resellers, or dangerous sub-potent or super-potent products that were improperly manufactured.
Efforts of federal and state authorities have kept infiltration of counterfeit drugs in the U.S. drug supply chain to a minimum. Our success is a result of the extensive system of laws and our enforcement efforts. In recent years, however, FDA is challenged by efforts of increasingly well-organized counterfeiters who are often located overseas, backed by sophisticated technologies and criminal operations, intent on profiting from drug counterfeiting at the expense of American patients. To respond to this domestic emerging threat, FDA has been working with manufacturers, wholesalers, retailers, other federal and state government entities, standard bodies, and others to implement measures to further secure our nation's drug supply.
When FDA learns of schemes intended to use the drug supply to harm U.S consumers, we actively work to prevent them to the fullest extent of the law. A recent case illustrates why American consumers ordering prescription drugs from Canadian sources cause FDA great concern. In August 2006, FDA advised consumers not to purchase prescription drugs from various websites, including www.RxNorth.com, that have orders filled by a firm in Manitoba, Canada, following reports of counterfeit versions of prescription drug products being sold by these companies to U.S. consumers. FDA is investigating these reports and is coordinating with international law enforcement authorities on this matter. Laboratory results to date have found counterfeits from these websites, destined for the U.S. market, of the following drug products: Lipitor, Diovan, Actonel, Nexium, Hyzaar, Ezetrol (known as Zetia in the United States), Crestor, Celebrex, Arimidex, and Propecia.
In addition, just last month, FDA issued an alert to consumers who placed orders for specific drug products over the Internet (Ambien, Xanax, Lexapro, and Ativan), but instead received a product that, according to preliminary analysis, contains haloperidol, a powerful anti-psychotic drug. Reports show that several consumers in the U.S. have sought emergency medical treatment, after ingesting the suspect product, for symptoms such as difficulty in breathing, muscle spasms, and muscle stiffness. Haloperidol can cause muscle stiffness and spasms, agitation, and sedation. Identifying the actual sellers or websites has been challenging because of the deceptive practices of many commercial outlets on the Internet. Currently, the origin of these tablets is unknown but the packages were postmarked in Greece. Preliminary investigation has identified some of the responsible websites and we are currently pursuing both domestic and foreign leads. Details of additional cases are included in an appendix to this testimony.
In an effort to gauge the volume and scope of drugs coming into the U.S., we routinely survey international mail facilities. A recent finding confirms our concern that buying drugs from foreign sources pose specific risks to U.S. citizens. An FDA operation in 2005, called "Bait and Switch," found that nearly half of the imported drugs that FDA intercepted from four selected countries (India, Israel, Costa Rica, and Vanuatu) were shipped to fill orders that consumers believed were placed with "Canadian" pharmacies. Of the drugs being promoted as "Canadian," 85 percent appeared to come from 27 countries around the globe. Many of these drugs were not adequately labeled to help assure safe and effective use and some were found to be counterfeit.
FDA also works with U.S. Customs and Border Protection on their surveys at international mail facilities. In the last six months, FDA has assisted in two such operations. These operations revealed that we are still fighting the same issues we have seen in the past:
Last year, an FDA investigation found that many foreign medications, although marketed under the same or similar-sounding brand names as those in the U.S., contain different active ingredients than the U.S. products. For example, in the U.S., "Flomax" is a brand name for tamsulosin, a treatment for an enlarged prostate, while in Italy, the active ingredient in the product called "Flomax" is morniflumate, an anti-inflammatory drug.
FDA also has found 105 U.S. drug brand names that are so similar to drugs marketed in foreign countries that consumers who fill such prescriptions abroad may receive a drug with the wrong active ingredient. For example, in the United Kingdom, "Ambyen," a brand name for a drug product containing amiodarone, used to treat life-threatening abnormal heart rhythms, could be mistaken for "Ambien," a U.S. brand name for a sleeping pill. Consumers taking medications containing active ingredients not prescribed by their physician increase their risks of unnecessary side effects and possibly serious adverse outcomes.
FDA also publishes Import Alerts to field personnel about potentially dangerous drugs being offered for import into the U.S. Field personnel use this information to halt shipments of potentially dangerous products at the borders. For example, last month FDA added 39 known foreign suppliers of unapproved isotretinoin (known by the brand name Accutane) to an existing Import Alert, "Unapproved New Drugs Promoted in the U.S." The unsupervised use of isotretinoin carries significant potential risks, including birth defects and even fetal death, and may cause serious mental health problems. For this reason, the approved medication should only be taken by persons taking part in a specific risk management program closely monitored by their personal physician. Consumers receiving isotretinoin from these foreign sources are not likely taking part in the risk management program.
As a public health agency, we understand the importance of protecting the public health not only through regulation and enforcement, but also through education and collaboration. FDA's website (www.fda.gov) contains extensive information for consumers about drug importation, buying drugs online; counterfeit drugs, enforcement activities, potential public health threats, as well as resources to report problems with FDA regulated products or websites that could be selling fake or harmful products.
FDA coordinates with other governmental bodies and meets regularly with other federal agencies and state officials to share information and identify opportunities for partnering in enforcement actions. Some of the federal agencies that are FDA partners include U.S. Customs and Border Protection, U.S. Drug Enforcement Administration, U.S. Immigration and Customs Enforcement, U.S. Postal Service, and the Federal Bureau of Investigation, just to name a few. We also work with organizations representing consumers, health care practitioners, industry, and others. These relationships are essential to keep the Agency abreast of emerging issues, to leverage resources, and to best protect American consumers.
FDA understands that Congress and the public are concerned about the high cost of prescription drugs. FDA currently has an efficient generic drug approval program that brings lower cost versions of brand name drugs to U.S. consumers. In most instances, FDA-approved generic drugs are less expensive than generics sold abroad.
Prompt approval of generic drug product applications is a priority for FDA. Resources for generic drug approvals have consistently increased. Moreover, both the number of generic drug applications FDA receives and the number of applications FDA's Office of Generic Drugs (OGD) approves continue to increase each year as well. OGD recently instituted many new practices and procedures to help expedite the generic drug application review process. Because of these efforts, on the very day that the last controlling patents or exclusivities expired on an innovator product, OGD has approved at least one generic drug application in most cases. Recent examples of approvals when the exclusivities expired include pravastatin (Pravachol); sertraline (Zoloft); simvastatin (Zocor); and ondansetron (Zofran). Multiple versions of these products from various manufacturers are currently on the market.
Last year, 21 applications for meloxicam (Mobic), a product with no patent or exclusivity protection blocking approval, were approved. (This product is used to relieve the signs and symptoms of osteoarthritis and rheumatoid arthritis.) The cost to consumers of this product dropped dramatically after these generic approvals. Using OGD's "cluster" team approach, many of these applications were approved in just over nine months. These approvals will result in many generic alternatives available for patients potentially saving millions of dollars in medication costs for consumers and the Federal government.
The standards for drug review and approval in the U.S. are the best in the world, and the safety of our drug supply mirrors these high standards. However, despite the very real risks, a substantial number of Americans are obtaining prescription medications from foreign sources. U.S. consumers often seek out Canadian suppliers, sources that purport to be Canadian, or other foreign sources that they believe to be reliable. Many drugs purported to be from Canada are actually from other foreign countries that lack regulatory oversight and FDA cannot assure the safety or effectiveness of these drugs.
Thank you for the opportunity to testify. I look forward to responding to any questions you may have.
![]()
Provided below are summaries of selected cases FDA investigated that pertain to drug importation.
Counterfeit Percocet®, Viagra® and Cialis® Tablets: In January 2007, an individual in Philadelphia who purchased thousands of counterfeit drugs over the Internet from China, including Percocet, Viagra and Cialis, was sentenced in the Eastern District of Pennsylvania on charges related to trafficking in counterfeit goods and other counterfeit prescription drug related charges. The defendant sent samples of various medications to a counterfeit pharmaceutical manufacturer in China to be copied and manufactured. After the counterfeits were made in China, the medication was then shipped to the defendant in Philadelphia for eventual sale on the Internet and other venues. The United States Attorney said of this case: "When you go around government safeguards to either the Internet or the street to purchase prescription medication, you have no idea what you're getting. The reality is that you might wind up taking something that is ineffective, as we saw in this case, or downright dangerous."
This Office of Criminal Investigations (OCI) case, worked jointly with U.S. Immigration and Customs Enforcement (ICE), U.S. Drug Enforcement Administration (DEA), U.S. Postal Inspection Service and the Philadelphia Police Department, was part of a much larger OCI-ICE counterfeit drug investigation.
Clandestine Drug Manufacturing of Internet Drugs: In 2006, eleven individuals and an Atlanta, Georgia-based company were indicted by a federal grand jury on multiple felony charges relating to a scheme to sell adulterated and unapproved new drugs over the Internet. The defendants in this case opened up a pharmaceutical manufacturing facility in Belize where they made over 24 different prescription medications. The defendants marketed the drugs through "spam" e-mail advertisements where they claimed the drugs were Canadian generic versions of brand name drugs. Some of the drugs the defendants made were unapproved versions of Ambien®, Valium®, Xanax®, Cialis®, Lipitor®, Vioxx® and others. These drugs were then purchased by and shipped to U.S. consumers and to various drug wholesalers.
 |
 |
Dextromethorphan deaths: On April 12, 2006, two men were sentenced in the Southern District of Indiana Federal Court to 77 months incarceration after pleading guilty to introducing a misbranded drug into interstate commerce; specifically, dextromethorphan (DXM), a cough suppressant, which they sold over the Internet through their website.
This case started in 2005 after five young people died after ordering and consuming DXM from the defendant's website. DXM is an anti-tussive (cough suppressant), which is approved for over-the-counter cough medications. The defendants purchased the raw ingredients from a firm in India, manufactured the finished product, and sold the DXM through their website. The defendants marketed the DXM by falsely claiming that DXM was a chemical used for research and development rather than a drug for human consumption. DXM is often abused by some in order to experience a "high."
 |
 |
Counterfeit Viagra®, Cialis®, and Lipitor®: In January 2006, an individual from the state of Washington was convicted for his involvement in the importation of counterfeit drugs from China including Viagra, Cialis and Lipitor and the subsequent distribution of those counterfeit drugs. In this joint OCI, ICE investigation, cooperation was sought and received from the Chinese government. As a result of this cooperation, the Chinese authorities arrested eleven individuals in China and recovered significant amounts of counterfeit drugs and counterfeit drug packaging. The defendant was sentenced in October 2006 to ten month's incarceration.

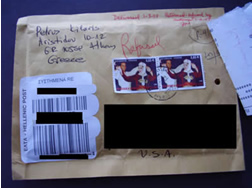
Consumers Warned of Receiving Incorrect Medication in the Mail: In February 2007, FDA issued an alert to consumers who placed orders for various medications such as Xanax®, Ativan®, Lexapro®, and Ambien®, over the Internet. Instead of receiving the products they ordered, these consumers instead received a product containing haloperidol, a powerful anti-psychotic. Some of these consumers sought emergency medical treatments for a variety of symptoms after ingesting the suspect product. In all instances, consumers received the suspect medication in packaging, which was postmarked from Greece. FDA is attempting to identify the actual vendors and source of the suspect medication, but the illusive nature of the Internet and the deceptive practices of many Internet pharmaceutical businesses are making identification of the actual supplier of these medications problematic. This investigation is ongoing.
 |
 |
Counterfeit Drug Arrest in Hong Kong: In September
2006, an individual from China was arrested by officers of the Hong Kong Customs
and Excise Department based on a federal arrest warrant issued by the U.S.
District Court for the District of Colorado. The defendant was
arrested in Hong Kong after meeting with an undercover OCI agent who posed
as a buyer of over 400,000 counterfeit Cialis and Viagra tablets. This
investigation also involved the sale of several thousand counterfeit Tamiflu® capsules
that were manufactured in China and shipped to the U.S. Information
developed by OCI and ICE was shared with Chinese authorities, which led to
the August 2006 arrests of four individuals in China. Furthermore,
information developed during this joint OCI-ICE counterfeit drug investigation
was the basis for the previously mentioned counterfeit Percocet investigation
in Philadelphia, PA. In addition to the arrest in Hong Kong, three
other defendants in the U.S. have pled guilty to counterfeit drug charges. This
case is ongoing.
Counterfeit Viagra® and Cialis®: In July 2006,
a man was arrested by OCI and ICE agents after several transactions in the Houston,
Texas area where significant quantities of counterfeit Viagra were sold to an
undercover ICE agent. Subsequent to the arrest, counterfeit Viagra
and Cialis valued at approximately $600,000 were seized. The drugs
were manufactured in China and sent to the suspect in Houston for distribution. The
suspect was charged with trafficking in counterfeit goods and other related counterfeit
drug charges and remains incarcerated as a potential flight risk. The
defendant pled guilty in this case in October 2006 but has not yet been sentenced. Other
defendants have been arrested and are awaiting judicial action. This
joint OCI-ICE investigation is ongoing.
FDA Warns Consumers of Canadian Website Shipping Counterfeit Medications: In August 2006, FDA published a warning to consumers about counterfeit medications shipped from RxNorth, a company based in Manitoba, Canada, which operates several websites. RxNorth, which operates as Mediplan Prescription Plus Pharmacy and Mediplan Global Health, were shipping counterfeit medications from various countries to American consumers who were ordering a variety of medications through the RxNorth and affiliated websites. Although this is an ongoing investigation, FDA issued a press release alerting consumers about these websites because of the potential dangers of counterfeit medications.
Counterfeit Lipitor® Tablets: In August 2005 in the Western District of Missouri, three businesses and eleven individuals were indicted for their involvement in a $42 million conspiracy to sell counterfeit, smuggled and misbranded Lipitor and other drugs and for participating in a conspiracy to sell stolen drugs. These indictments are the result of an ongoing OCI investigation that was begun by OCI in April 2003 involving the manufacturing, smuggling, and the interstate distribution of counterfeit pharmaceuticals. To date, twelve defendants have been convicted; one received a nine- year term of imprisonment. Additional defendants are awaiting trial.
![]() Get free
weekly updates about FDA press releases, recalls, speeches, testimony
and more.
Get free
weekly updates about FDA press releases, recalls, speeches, testimony
and more.
![]()