

 |
 |
FDA Home Page | Search
FDA Site | FDA A-Z Index | Contact
FDA
![]()

U. S. Department of Health and Human Services
Food and Drug Administration
Rockville, Maryland 20857
![]()
COMBATING COUNTERFEIT DRUGS: A REPORT OF THE FOOD AND DRUG ADMINISTRATION: February 2004
A. Purpose of the Anti-Counterfeiting Initiative
B. Scope of the Problem
C. What is in this Report
D. Securing our Nation's Drug Supply
a. Unit of Use Packaging
b. Tamper Evident Packaging
c. Authentication Technology
d. Identification of Products likely to be counterfeited
e. Radio-frequency Identification (RFID) Technology
2. REGULATORY INITIATIVES AND STATE MODEL RULES
A. PRESCRIPTION DRUG MARKETING ACT (PDMA)
B. MODEL RULES FOR WHOLESALE DISTRIBUTOR LICENSING STRENGTHENED
C. HIGHER PENALTIES FOR DRUG COUNTERFEITING
3. CREATION OF A COUNTERFEIT ALERT NETWORK FOR INFORMATION DISSEMINATION AND EDUCATION
4. HEALTH PROFESSIONAL REPORTING ENCOURAGED VIA MEDWATCH
6. FDA'S RAPID RESPONSE TO REPORTS OF SUSPECT COUNTERFEIT DRUGS STREAMLINED
7. EDUCATING THE PUBLIC AND HEALTH PROFESSIONALS
a. Consumers
b. Pharmacists and Other Health Care Professionals
APPENDIX A: COUNTERFEIT ALERT NETWORK CO-SPONSORSHIP AGREEMENT
APPENDIX B: EXPANDED DESCRIPTION OF COMMENTS RECEIVED
The counterfeiting of currency and consumer products are common problems that plague governments and manufacturers around the world, but the counterfeiting of medications is a particularly insidious practice. Drug counterfeiters not only defraud consumers, they also deny ill patients the therapies that can alleviate suffering and save lives. In some countries the counterfeiting of drugs is endemic -- with some patients having a better chance of getting a fake medicine than a real one. In many more countries, counterfeit drugs are common. In the United States, a relatively comprehensive system of laws, regulations, and enforcement by Federal and state authorities has kept drug counterfeiting rare, so that Americans can have a high degree of confidence in the drugs they obtain through legal channels. In recent years, however, the FDA has seen growing evidence of efforts by increasingly well-organized counterfeiters backed by increasingly sophisticated technologies and criminal operations to profit from drug counterfeiting at the expense of American patients.
To respond to this emerging threat, Commissioner of Food and Drugs Mark McClellan formed a Counterfeit Drug Task Force in July 2003. That group received extensive comment from security experts, Federal and state law enforcement officials, technology developers, manufacturers, wholesalers, retailers, consumer groups, and the general public on a very broad range of ideas for deterring counterfeiters. Those comments reinforced the need for FDA and others to take action in multiple areas to create a comprehensive system of modern protections against counterfeit drugs. FDA discussed those ideas, and considered alternatives and criticisms at its public meetings, to develop a comprehensive framework for a pharmaceutical supply chain that will be secure against modern counterfeit threats. The specific approach to assuring that Americans are protected from counterfeit drugs includes the following critical elements:
Because the capabilities of counterfeiters continue to evolve rapidly, there is no single "magic bullet" technology that provides any long-term assurance of drug security. However, a combination of rapidly improving "track and trace" technologies and product authentication technologies should provide a much greater level of security for drug products in the years ahead. Similar anti-counterfeiting technologies are being used in other industries, and FDA intends to facilitate their rapid development and use to keep drugs secure against counterfeits.
a. The adoption and common use of reliable track and trace technology is feasible in 2007, and would help secure the integrity of the drug supply chain by providing an accurate drug "pedigree," which is a secure record documenting the drug was manufactured and distributed under safe and secure conditions.
Modern electronic technology is rapidly approaching the state at which it can reliably and affordably provide much greater assurances that a drug product was manufactured safely and distributed under conditions that did not compromise its potency. FDA has concluded that this approach is a much more reliable direction for assuring the legitimacy of a drug than paper recordkeeping requirements, which are more likely to be incomplete or falsified, and that it is feasible for use by 2007. Radiofrequency Identification (RFID) tagging of products by manufacturers, wholesalers, and retailers appears to be the most promising approach to reliable product tracking and tracing. Significant feasibility studies and technology improvements are underway to confirm that RFID will provide cost-reducing benefits in areas such as inventory control, while also providing the ability to track and trace the movement of every package of drugs from production to dispensing. Most importantly, reliable RFID technology will make the copying of medications either extremely difficult or unprofitable. FDA is working with RFID product developers, sponsors, and participants of RFID feasibility studies to ensure that FDA's regulations facilitate the development and safe and secure use of this technology. FDA is also working with other governmental agencies to coordinate activities in this area.
b. Authentication technologies for pharmaceuticals have been sufficiently perfected that they can now serve as a critical component of any strategy to protect products against counterfeiting.
Authentication technologies include measures such as color shifting inks, holograms, fingerprints, taggants, or chemical markers embedded in a drug or its label. The use of one or more of these measures on drugs, starting with those considered most likely to be counterfeited, is an important part of an effective anti-counterfeiting strategy. Because counterfeiters will adapt rapidly to any particular measure and because the most effective measures differ by product, the most effective use of authentication technology will vary by drug product over time. FDA intends to clarify its policies and procedures to help manufacturers employ and update these technologies safely and effectively. In particular, FDA plans to publish a draft guidance on notification procedures for making changes to products (e.g., addition of taggants), their packaging, or their labeling, for the purpose of encouraging timely adoption and adaptation of effective technologies for detecting counterfeit drugs. FDA also intends to continue to evaluate and provide information to stakeholders on forensic technologies (e.g., use of product fingerprinting, addition of markers) and other analytical methods that allow for rapid authentication of drug products. FDA also plans to support the development of criteria that contribute to counterfeiting risk, and/or the development of a national list of drugs most likely to be counterfeited based on these criteria, to assist stakeholders in focusing their use of anti-counterfeiting technologies as effectively as possible.
At the time PDMA was enacted the only way to pass on a pedigree for drugs was to use paper, which has posed practical and administrative challenges. RFID technology, which would provide a de facto electronic pedigree, could surpass the intent of PDMA and do so at a lower cost. In light of the rapid progress toward much more effective electronic pedigrees that can be implemented within several years, FDA intends to continue to stay its regulations regarding certain existing pedigree requirements to allow suppliers to focus on implementing modern effective pedigrees as quickly as possible.
Because states license and regulate wholesale drug distributors they have an important role in regulating the drug distribution supply chain. The FDA is working with the National Association of Boards of Pharmacy on its effort to develop and implement revised state model rules for licensure of wholesale drug distributors. Such rules will make it difficult for illegitimate wholesalers to become licensed and transact business, thus making it easier to deter and detect channels for counterfeit drugs. Some states have already reduced counterfeit threats by adopting such measures. FDA will continue working with NABP and states to facilitate adoption of the Model Rules.
Although increased criminal penalties would not affect FDA's regulatory framework for overseeing the U.S. drug supply, they would provide an added deterrent to criminals who work to counterfeit our citizens' medications. FDA has requested that the United States Sentencing Commission amend the sentencing guidelines to increase substantially the criminal penalties for manufacturing and distributing counterfeit drugs and to provide for enhanced penalties based on the level of risk to the public health involved in the offense.
Effective protection against counterfeit drugs includes actions by drug producers, distributors, and dispensers to secure their business practices such as ensuring the legitimacy of business partners and refusing to do business with persons of unknown or dubious background, taking steps to ensure physical security, and identifying an individual or team in the organization with primary responsibility for ensuring that effective security practices are implemented. The wholesalers have already drafted a set of secure business practices and FDA will continue to work with other major participants of the drug supply chain to develop, implement, and disseminate such business practices, through such steps as issuing guidance and supporting the development of industry best practices. To help ensure secure business practices, FDA intends to increase its inspection efforts of re-packagers whose operating procedures place them at increased risk for the introduction of counterfeit drugs.
If counterfeit drugs do enter the American marketplace, procedures should be in place to recognize the hazard and alert the public quickly and effectively. FDA plans to take new steps to encourage health professionals to report suspected counterfeit drugs to FDA's MedWatch system. FDA also intends to create a Counterfeit Alert Network to provide timely and effective notification to affected health professionals and the public whenever a counterfeit drug is identified.
FDA will develop educational materials, including new tools on the FDA website at www.fda.gov, new public service announcements, and new educational partnerships with consumer and health professional organizations, to help consumers avoid counterfeits. FDA will enhance its educational programs for pharmacists and other health professionals about their role in minimizing exposure to, identifying, and reporting counterfeits.
Counterfeit drugs are a global challenge to all nations, and criminal counterfeiting operations are increasingly operating across national borders. FDA intends to work with the World Health Organization, Interpol, and other international public health and law enforcement organizations to develop and implement worldwide strategies to combat counterfeit drugs.
The steps described in this report are intended to secure the safety and of the U.S. drug supply, which the FDA regulates. The FDA does not have the legal authority or resources to assure the safety and efficacy of drugs purchased from other countries outside our domestic drug distribution system, or from unregulated Internet sites that are not run by pharmacies licensed and regulated by U.S. states.
The actions described in this report are based on the work of an internal FDA Counterfeit Drug Task Force,1 which was formed in July 2003 by Commissioner of Food and Drugs Mark McClellan, M.D., Ph.D., with the goals of:
FDA believes that counterfeiting is not widespread within the system of manufacturing and distributing pharmaceuticals legally in the United States, as a result of an extensive system of federal and state regulatory oversight and steps to prevent counterfeiting undertaken by drug manufacturers, distributors, and pharmacies. However, the agency has recently seen an increase in counterfeiting activities as well as increased sophistication in the methods used to introduce finished dosage form counterfeits into the otherwise legitimate U.S. drug distribution system. FDA counterfeit drug investigations have increased to over 20 per year since 2000, after averaging only 5 per year through the late 1990's. (See Figure 1 -- Chart of FDA investigations) Increasingly, these investigations have involved well-organized criminal operations that seek to introduce finished drug products that may closely resemble legitimate drugs yet may contain only inactive ingredients, incorrect ingredients, improper dosages, sub-potent or super-potent ingredients, or be contaminated. Thus, drug counterfeiting poses real public health and safety concerns today, and may pose an even greater threat in the future if we fail to take preventative measures now. As counterfeiters continue to seek out new technologies to make deceptive products and introduce them into legitimate commerce, our systems for protecting patients must respond effectively.
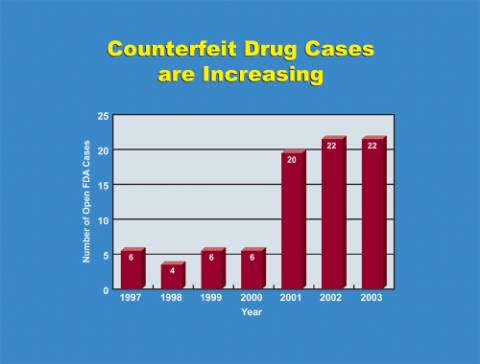
Fig. 1: FDA open investigations 1997-2003
Although exact prevalence rates in the U.S. are not known, outside the U.S.
drug counterfeiting is known to be widespread and affect both developing and
developed countries. In some countries more than half of the drug supply may
consist of counterfeit drugs. For example, recent reports have detailed that
more than 50% of anti-malarials in Africa are believed to be counterfeit. In
virtually all countries, counterfeit drug operations have been uncovered in
recent years.
The body of this report contains a range of findings that have broad support from industry stakeholders and the public to identify and address the vulnerabilities in the U. S. drug distribution system to counterfeit drugs.
This report is based on the potential options discussed in the Task Force's Interim Report, the comments FDA received in response to that report, our internal discussions, and on information gathered and reviewed by the Task Force including:
Appendix A contains the Counterfeit Alert Network Co-sponsorship agreement. See www.fda.gov/oc/initiatives/counterfeit/archive.html for background information that was included in the Task Force's Interim Report (released on October 2, 2003) as well as a detailed discussion of the comments FDA received. Appendix B contains a more detailed discussion of the comments FDA received and considered in developing the final report.
The FDA is grateful for the input and universal support, not only with regard to the creation of the task force, but also with regard to the need for securing the nation's drug supply.
To secure the U. S. drug supply chain, there are several areas that deserve attention, including the areas of technology, business practices, legislation, regulation, public awareness and education, creation of an alert network, and international cooperation.
1) What FDA sought comment on:
2) What the comments said:
Comments cited a large number of benefits, including eliminating the need for re-packaging and improved patient compliance, as well as a large number of costs, including those associated with shifting production from bulk packaging. The cost hurdle to counterfeiters, created by unit of use packaging, was said not to be high enough for it to be effective as a stand-alone anti-counterfeiting measure. A detailed discussion of the comments is in Appendix B.
3) Discussion:
Although single unit containers (e.g., blister packs) usually come to mind, unit of use packaging is any container closure system designed to hold a specific quantity of drug product for a specific use and dispensed to a patient without any modification except for the addition of appropriate labeling.
Unit of use packaging does not create a sufficiently high level of security to justify its use as a stand-alone anti-counterfeiting measure. However, because of its many other benefits, which may vary on a product specific basis (e.g., tablets, liquid forms), manufacturer initiated cost-benefit analyses of particular products, starting with newly approved products and products that are likely to be counterfeited, are likely to show that unit of use packaging could be effective as one layer in a multi-layered anti-counterfeiting strategy.
|
Unit of use packaging can be beneficial in fighting counterfeit drugs.
|
The comments on tamper evident packaging mirrored the comments on unit of use packaging.
Decisions to employ tamper evident packaging on prescription drug containers as an anti-counterfeiting measure require a product specific cost-benefit analysis. As with unit of use packaging, FDA does not believe that tamper evident packaging presents a high enough hurdle for counterfeiters to make it effective as a stand-alone anti-counterfeiting measure.
Tamper evident packaging may be beneficial in fighting counterfeiting of prescription drugs.
|
The comments stressed that there was no "silver bullet" anti-counterfeiting technology because sophisticated, well-financed counterfeiters can defeat any anti-counterfeiting measure. Therefore, the best strategy is to use multiple, periodically changing, authentication measures on a product specific basis after doing a risk analysis that takes into account the risk that the product will be counterfeited and the public health risk if the product is counterfeited.
Given the rapid developments in anti-counterfeiting technology and the dangers of aiding counterfeiters by locking in or requiring certain technologies, most comments stressed that the FDA should not mandate the use of specific anti-counterfeiting technologies.
FDA issuance of guidance concerning the agency's application and notification policies and procedures related to incorporating anti-counterfeiting measures into products (e.g., taggants) or labeling and packaging (e.g., inks, holograms) was universally supported.
A detailed discussion of the comments is in Appendix B.
FDA agrees that the danger of unwittingly assisting counterfeiters and stifling technologic development outweigh the benefits that would accrue if it were to mandate the use of a specific authentication technology at this time. Furthermore, the decision to deploy authentication technologies is best made by the manufacturer, based on a product specific risk-benefit analysis that, in the future, should take into account whether mass serialization and radio-frequency identification technology (see below) is being used for tracking and tracing the drug.
However, due to the high costs and technical barriers that authentication technologies create for counterfeiters, their use is a critical component of any effective multi-layered anti-counterfeiting strategy, especially for products that are likely to be counterfeited. Therefore, FDA believes that an appropriate role for it is to facilitate the use of authentication technologies by reducing any regulatory hurdles that may exist relating to their use.
|
Existing authentication technologies have been sufficiently perfected they can now serve as a critical component of any strategy to protect products against counterfeiting.
|
d. Identification of Products likely to be counterfeited
Although a few comments suggested that all products were at high risk for being counterfeited, most of the comments FDA received supported the idea of developing criteria by which stakeholders could determine which products are likely to be counterfeited and/or developing a national list of products likely to be counterfeited based on these criteria. There was general agreement that the existence of state specific lists, each with its own regulatory requirements, could inhibit commerce and adversely affect the availability of drugs. FDA notes that the State of Florida has already published a list of "specified products" (i.e., a list of drugs most likely to be counterfeited) that is being used to implement state pedigree requirements. A detailed discussion of the comments is in Appendix B.
Due to the large number of drugs with the potential to be counterfeited, FDA does not believe it is possible to create a comprehensive list of all such drugs. However, FDA does believe that a national list of those drugs most likely to be counterfeited and/or a set of criteria to use for determining those drugs would be useful for stakeholders to use at their discretion. Uses could include:
FDA strongly supports the development of such a set of criteria, or a list based on these criteria, that has the support and participation of all stakeholders. Regular input from interested parties as well as the ability to add or delete drugs from the list on short notice are important parts of the process.
FDA believes that members of regulated industry are better positioned at this time than FDA to develop a process for creating, maintaining, and updating such a list (and/or set of criteria).
|
FDA has concluded that there would be great value in the creation of a national list of drugs most likely to be counterfeited based on factors that are likely to contribute to counterfeiting risk.
|
FDA is aware of only one national list of drugs most likely to be counterfeited. The list was developed by the National Association of Boards of Pharmacy and is available at www.nabp.net.
e. Radio-frequency Identification (RFID) Technology
There was universal support for the adoption of electronic track and trace technology. RFID was cited as being the technology with the strongest potential for securing the supply chain but that it was not ready for widespread commercial use with pharmaceutical products. Many costs, potential benefits, and unresolved issues related to RFID were cited. The potential benefits included the ability to control inventory and conduct rapid, efficient recalls, while costs that could hinder the adoption of RFID included purchase of tags and other hardware, integration into existing information systems, and compliance with regulatory requirements (e.g., labeling, electronic records). Important unresolved issues included the need to develop standards and business rules for RFID, the need to address database management issues, and the need to determine the effect of RFID on product quality.
FDA was also informed that some companies are planning feasibility studies concerning business uses of RFID for early this year and that other activities related to creating standards, business rules, and migratory pathways for RFID are also ongoing. A detailed discussion of these activities and other comments concerning RFID is in Appendix B.
Use of mass serialization to uniquely identify all drug products intended for use in the United States is the single most powerful tool available to secure the U. S. drug supply. Mass serialization involves assigning a unique number (the electronic product code or EPC) to each pallet, case, and package of drugs and then using that number to record information about all transactions involving the product, thus providing an electronic pedigree from the point of manufacture to the point of dispensing. This unique number would allow each drug purchaser to immediately determine a drug's authenticity, where it was intended for sale, and whether it was previously dispensed.
Although there is general agreement that widespread use of mass serialization is inevitable, several important issues remain unresolved, including the migratory path(s) that participants in the drug distribution system will follow as they begin to serialize their products, and the most likely timeline for widespread commercial use.
It currently appears that the technology most likely to bring mass serialization into widespread commercial use by the pharmaceutical industry is RFID, although two-dimensional bar codes may be used for some products. RFID technology includes not only the silicon tags containing the EPC, but also antennas, tag readers, and information systems that allow all users to identify each package of drugs and its associated data. This data can be used not only to authenticate drugs but also to manage inventory, conduct rapid, targeted recalls, prevent diversion, and ensure correct dispensing of prescriptions.
Acquiring and integrating RFID technology into current manufacturing, distribution, and retailing processes will require considerable planning, experience, and investment of resources. Currently, some manufacturers, wholesalers, and retailers are developing business plans and testing mass serialization using RFID while others are taking a wait and see approach. Due to rapid technologic advancements, the lack of significant market place experience with it in the pharmaceutical supply chain, each participant is best situated to determine his optimal path(s) to adopting it.
Therefore, FDA has identified near term actions, described below, for it to take in order to facilitate the performance of mass serialization feasibility studies using RFID, and to assist stakeholders as they migrate towards the use of RFID technology.
In the long term, after there is significant market place experience with RFID, FDA plans to propose or clarify, as necessary and appropriate, policies and regulatory requirements relating to the use of RFID. Labeling, electronic records, product quality, and Current Good Manufacturing Practices (cGMP) requirements are issues that have arisen in connection with RFID. However, regulatory or policy determinations regarding these, or other, issues should not be made until they can be informed by sufficient data and significant marketplace experience with RFID. FDA has also identified a series of actions, discussed below, that would help industry stakeholders and standard-setting organizations achieve this goal.
Lastly, stakeholders will need to ensure that they comply with the patient privacy protections provided by the Health Insurance Portability and Accountability Act as they implement use of RFID technology.
|
The adoption and common use of RFID as the standard track and trace technology, which is feasible in 2007, would provide better protection.
|
Each industry stakeholder interested in implementing RFID would benefit from the following steps:
To the extent possible, it would be most useful for interested firms to perform these actions concurrently. For example, standards development requires knowledge gained from feasibility studies in order to move forward, and vice versa.
Any effort to develop standards for mass serialization of pallets, cases, and packages would be most effective if it addressed the following issues:
All levels of government, in addition to the private sector, should take responsibility for ensuring the safety and security of the U.S. drug distribution system. Each level has a role in deterring and preventing the introduction of counterfeit drugs into the nation's drug supply chain. To complement and build on the technology measures described above, regulatory and legislative steps at all levels of government may be necessary. At the Federal level, FDA is taking steps to meet the objectives of the Prescription Drug Marketing Act (PDMA), which is intended to address vulnerabilities in the U.S. drug distribution system. At the State level, it would be beneficial for states to strengthen their provisions governing wholesale distribution, as described below in the revised Model Rules for Licensure of Wholesale Distributors. And, FDA plans to pursue increased criminal penalties for counterfeiting in the United States Sentencing Commissions sentencing guidelines.
A. PRESCRIPTION DRUG MARKETING ACT (PDMA)
Many of the comments that discussed PDMA acknowledged the limitations and concerns of full implementation of PDMA. However, many comments also supported the use of paper pedigrees for their deterrent value and as a means to verify prior sales through due diligence. A risk-based approach to implementing PDMA, which focuses on those drugs that are at high risk of being counterfeited, was suggested, as well as maintaining a full pedigree that documents all sales and transactions back to the manufacturer for drugs and high risk. One comment suggested an interim solution of "one forward, one back" pedigree for high-risk drugs. However, a number of the comments noted the high cost and incomplete protection provided by such paper requirements, especially as a general interim measure; by the time these costly requirements were phased in, they could be replaced by a more modern system. A majority of the comments supported the eventual use of an electronic pedigree for all drug products in the supply chain and indicated that an electronic pedigree should be considered as a modern solution to fulfilling and exceeding the PDMA goals, and urged FDA to take steps to help achieve a reliable pedigree solution as quickly as possible. As noted above, FDA believes that substantial progress toward a more cost-effective solution than incomplete and costly paper pedigrees is possible within the next several years. A detailed discussion of the comments is in Appendix B.
FDA has worked closely with affected parties to identify and resolve concerns related to the implementation of the pedigree requirements of the PDMA. Through the various public comment opportunities over the years, the agency has heard mixed reviews about the value, utility, and difficulty of implementing a paper pedigree that identifies each prior sale, purchase, or trade of such drug. The comments received in response to questions raised in the Interim Report confirm that these concerns continue.
FDA is encouraged by the enthusiasm and interest that stakeholders in the U.S. drug supply chain have expressed toward the adoption of sophisticated track and trace technologies that are more reliable than paper pedigrees. As discussed above, there appears to be movement by industry toward implementation of electronic track and trace capability in 2007. When this is in place, RFID should be able to function as a de facto electronic pedigree that follows the product from the place of manufacturer through the U.S. drug supply chain to the final dispenser. If developed properly, this electronic pedigree could be used to meet the statutory requirement in 21 U.S.C. § 353(e)(1)(A) to provide a pedigree under certain circumstances.
In the interim, until the electronic pedigree is in widespread use, voluntary
adoption of multi-layer strategies and measures discussed in this report would
reduce the likelihood that counterfeit drugs will be introduced into the U.S.
drug distribution system. These measures, combined with RFID technology, can
help provide effective long-term protections that will minimize the number of
counterfeit drug products in the U.S. distribution system.
As discussed in a notice published in the Federal Register in conjunction with
the publication of this report, FDA plans to continue to stay the of implementation
of 21 CFR §§ 203.3(u) and 203.50. However, the agency intends to
continue to reassess the stay of implementation on an annual basis. The agency
will monitor closely whether progress toward the implementation of electronic
pedigrees continues at the rapid pace evident in this task force analysis.
Our plan to reassess the stay annually is part of the agency's strong commitment
to see that effective product tracing is implemented as quickly as possible.
The agency also encourages wholesalers to provide pedigree information that
documents the prior history of a drug product, particularly for drugs most
likely to be counterfeited, even when the passing of such a pedigree is not
required by the Act. The suggestion from the comments that there be a one-forward,
one-back pedigree for high-risk drugs in the interim, until an electronic
pedigree is uniformly adopted, may have merit. However, FDA believes that
Congress would have to amend section 503(e) of the Act if such a system is
to become a requirement.
|
Adoption of electronic track and trace technology would help stakeholders meet and surpass the goals of PDMA. Therefore, FDA intends to focus its efforts on facilitating industry adoption of this technology within the next few years.
|
B. MODEL RULES FOR WHOLESALE DISTRIBUTOR LICENSING STRENGTHENED
The comments overwhelmingly supported strengthening state requirements governing the licensure and oversight of wholesale distributors. Many comments cited the systemic weaknesses in the oversight of the wholesale drug industry and that existing inspection and due diligence processes are often insufficient to detect criminal activity. Some comments noted the positive steps already taken by some states, such as Florida, toward more effective regulation of wholesale distributors. For example, Florida has implemented more stringent requirements for licensure, stronger penalties, and due diligence requirements. Most comments stated that the full adoption of revised NABP model rules would improve security nationwide, and that stricter uniform standards were desirable across all 50 states so as not to create 50 different sets of criteria and rules for licensing. FDA was encouraged to revisit the current minimum standards requirements described in 21 CFR Part 205 to assess whether a 'federal floor' for states would enhance or diminish state efforts to meet the NABP recommendations. A detailed discussion of the comments is in Appendix B.
FDA is pleased to recognize the recent efforts by NABP in revising the Model
Rules. The revised Model Rules significantly strengthen the requirements for
licensure, as well as put in place or fortify requirements that will ensure
and protect the integrity of drug products as they travel through the U.S. drug
supply chain from the manufacturer to the consumer.
NABP sought comment from FDA, as well as interested stakeholders, in developing
the revised Model Rules. The comments that FDA received as part of the anti-counterfeiting
initiative have been discussed with NABP.
The revision of the Model Rules sought to enhance the protections included in the original version of the Model Rules and close existing gaps. The table below contains highlights of the revised Model Rules:
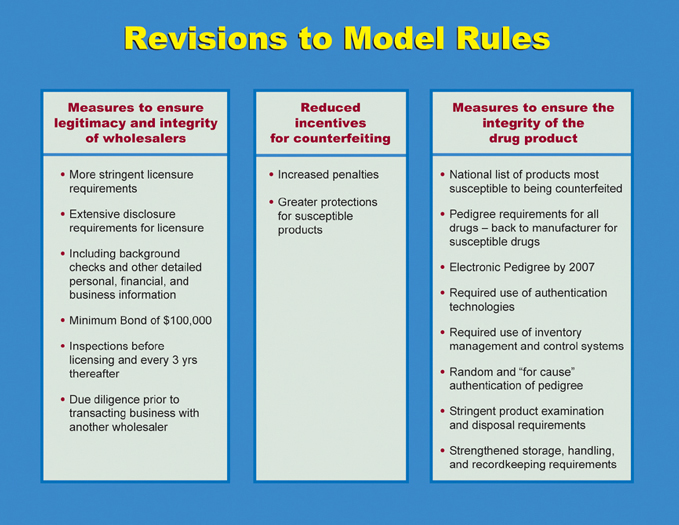
NABP is taking steps to facilitate implementation of the revised Model Rules,
including: 1) publishing a list of susceptible products and calling for a coalition
of national organizations to develop a process to maintain and update the list;
2) serving as bondholder for wholesalers in order to consolidate the need to
hold a bond in all states where a wholesaler may do business; and 3) establishing
a clearinghouse that will list wholesalers who receive accreditation by NABP
and who have passed an inspection by their newly created inspection service,
which NABP will conduct in partnership with the states. FDA supports NABP's
efforts to facilitate adoption and implementation of the enhanced Model Rules.
Counterfeiting is a problem that is not isolated to one state. If a state strengthens
its licensing requirements while a bordering state does not, the counterfeiters
and illegitimate wholesalers will likely move into the bordering state. Widespread
state adoption, implementation, and enforcement of the Model Rules would help
combat counterfeiting.
|
Because States have an important role in regulating drug distributors, adopting and enforcing stronger state anti-counterfeiting requirements would help in our collective effort to detect and deter counterfeiting.
|
There was overwhelming support and unanimous agreement that higher penalties for counterfeiting are needed.
FDA agrees with comments suggesting that higher penalties deter drug counterfeiters.
Current sentencing guidelines for counterfeit drug distribution are not commensurate with the public health threat posed by this criminal activity and strengthening the guidelines should help deter such conduct in the first instance. Despite the significant threat to public health posed by counterfeit drug products, current law provides penalties far below the level of some purely economic crimes. For example, counterfeiting a prescription drug label (bearing a registered trademark) is punishable by up to ten years in prison, while counterfeiting the drug itself is punishable by a maximum of only three years in prison. Therefore, FDA plans to continue to pursue its request that the United States Sentencing Commission consider amending the sentencing guidelines to substantially increase criminal penalties for manufacturing and distributing counterfeit drug products and to specifically provide for enhanced penalties based on the level of risk to the public health involved in the offense.
|
FDA intends to pursue its request that the United States Sentencing Commission consider amending the sentencing guidelines to increase substantially criminal penalties for manufacturing and distributing counterfeit drugs and to provide specifically for enhanced penalties based on the level of risk to the public health involved in the offense. |
3. CREATION OF A COUNTERFEIT ALERT NETWORK FOR INFORMATION DISSEMINATION AND EDUCATION
The agency received many comments supporting the creation of a counterfeit alert network. Most of the comments suggested that the agency take steps to build on existing networks and several comments offered their organizations' distribution lists or network as a conduit for the counterfeit alert network. The agency was advised that the counterfeit alert network should not be overused in order to avoid 'alert fatigue,' which could create indifference or doubt regarding the importance of the messages. The agency was encouraged to consider cost-effective public/private partnerships to design communication strategies and facilitate efforts to standardize anti-counterfeit communications and to augment and coordinate communication systems. A detailed discussion of the comments is in Appendix B.
The FDA is committed to informing the public, particularly consumers, pharmacists, other health professionals, wholesalers, and others involved in the U.S. drug distribution system, about counterfeit drug incidents in a timely manner. FDA is also committed to educating them about ways to identify and prevent counterfeits from entering into this system. To increase awareness of counterfeit drugs and safeguard the nations drug supply, FDA is creating a network of national organizations, consumer groups, and industry representatives to deliver time-sensitive messages and information about specific counterfeit incidents and educational messages about counterfeits in general. The network is called the "Counterfeit Alert Network."
Partners in the Counterfeit Alert Network will be required to enter into a co-sponsorship agreement with FDA that lays out roles and responsibilities. Partners agree to disseminate the FDA time-sensitive messages to their members/subscribers/readers in the manner outlined in the co-sponsorship agreement, to partner in delivering educational messages, and in the case of health professionals, provide a link to the MedWatch website to report suspect counterfeits. A copy of the co-sponsorship agreement can be found in Appendix C.
The agency plans to maintain a list (as it does now) of additional health professional, consumer, and industry organizations, and media outlets to notify when an actual counterfeit incident is confirmed and what steps to take to minimize risks and remove the product from the U.S. distribution system. This will help ensure the widest possible distribution to the appropriate audience(s).
FDA met with consumer groups, pharmacy groups, and physician groups to determine the type of information that would be most useful to receive from FDA in the event of a counterfeiting incident. FDA intends to create templates for standardizing the format and content of health professional and consumer information in the event of a counterfeit incident that can guide outreach efforts in an efficient manner, while assuring the flexibility FDA needs to formulate the messages.
|
FDA will create a Counterfeit Alert Network that links together and enhances existing counterfeit notification systems, to provide for timely and effective notification to health professionals and consumers of a counterfeit event.
|
Most of the comments supported the use of MedWatch for reporting suspect counterfeit drugs. These comments stated that health professionals are familiar with MedWatch and it would be too cumbersome and expensive to develop a new system, which people would have to be educated to use. One comment believed that reports of possible counterfeiting should be separate from MedWatch because it is not designed for criminal activity reporting and oversight. Another comment stated that because MedWatch is a voluntary reporting system, there could be significant under-reporting.
For nearly ten years, MedWatch has been FDA's reporting portal for adverse drug reactions and 'product problems.' These include problems with product quality that may occur during manufacturing, shipping, or storage, such as product contamination, defective components, poor packaging or product mix-up, questionable stability, and labeling concerns. If a pharmacist or consumer notices an unexplained change in size, shape, color, or taste of their dosage form, or notices that the coating is chipped or tablets are cracked, or that the drug is not working like it usually does, they may consider that to be a problem with their product. These are also characteristics that could occur if the product was a counterfeit drug. In fact, in the past, FDA has received some reports of suspect counterfeit drugs through MedWatch.
If a consumer suspects that his or her medicine is counterfeit, they are encouraged to contact the pharmacist who dispensed the drug, rather than report directly to MedWatch. The pharmacist may have information from the manufacturer that the shape, color, or taste of the product may have changed, or other information that may be helpful in determining if the product may be counterfeit or if the suspicious characteristic of the product or its packaging is expected.
The use of MedWatch is for health professional reporting. This would not affect the agreement with the Pharmaceutical Research and Manufacturers of America (PhRMA), whereby manufacturers have agreed to report counterfeits of their products to FDA's Office of Criminal Investigations, within 5 days of becoming aware of the counterfeit.
FDA has streamlined procedures for processing reports of suspect counterfeit drugs. The MedWatch Central Triage Unit (CTU) standard operating procedures (SOPs) have been amended to include "suspect counterfeit product" as a category of reports, so the CTU will know where to send the report for expedited processing.
It is easy and convenient to file a report with MedWatch. All reports are confidential and the identity of the reporter is not disclosed. FDA encourages reporting using the online reporting form that can be found at www.fda.gov/medwatch .
|
FDA plans to encourage and educate health professionals to report suspect counterfeit drugs to MedWatch.
|

The comments supported the need for development of secure business practices by all stakeholders in the drug distribution chain because each stakeholder has a responsibility to ensure that pharmaceutical products are authentic. The comments suggested that such practices include ensuring the legitimacy of business partners and refusing to do business with persons of unknown or dubious background, taking steps to ensure physical security, and identifying an individual or team in the organization with primary responsibility for ensuring that effective security practices are implemented.
It is critically important that the physical facilities involved in the production, distribution, or dispensing of pharmaceuticals are secure against counterfeit drugs. In the area of food safety, our Center for Food Safety and Nutrition (CFSAN) has issued guidance for the food industry on preventive measures that establishments may take to minimize the risk that products under their control will be subject to tampering or other malicious, criminal, or terrorist actions.
Although it was acknowledged that re-packagers were required to comply with Current Good Manufacturing Practices as set forth in 21 CFR 210 and 21 CFR 211, due to the involvement of re-packaging operations in some recent counterfeiting schemes, FDA was asked to provide more oversight and to conduct more frequent inspections of re-packagers.
See Appendix B for a detailed discussion of actions taken by manufacturers, wholesalers, and pharmacists to develop secure business practices.
Recent counterfeiting cases demonstrate that the current business practices of participants in the U. S. drug distribution system are in some cases inadequate to prevent the introduction of counterfeit drugs. Implementation of secure business practices by participants in the U.S. drug supply chain is critical for deterring and detecting counterfeit drugs. Therefore, FDA commends and strongly supports efforts to develop and implement secure business practices for these participants. FDA plans to facilitate and encourage the development of innovative approaches to securing business transactions in the drug supply chain. The number of stakeholders who have told FDA they are already implementing the business practices discussed above is very encouraging. In addition to identifying effective security measures, the designation of an individual or team to have primary responsibility for coordinating security activities helps ensure effective implementation.
FDA agrees that re-packaging operations can be a significant vulnerability in the drug supply chain. Although current statutory and regulatory requirements allow for appropriate oversight of re-packagers, FDA agrees that enforcement of those requirements could be strengthened.
|
For government efforts against counterfeit drugs to be successful, drug producers, distributors, and dispensers will have to take effective actions to secure their business practices.
|
6. FDA'S RAPID RESPONSE TO REPORTS OF SUSPECT COUNTERFEIT DRUGS STREAMLINED
The comments unanimously supported any efforts by the agency to rapidly respond to reports of suspect counterfeit drugs.
FDA takes reports of suspect counterfeit products very seriously. The agency is proud of its investigative tools and talents and its quick response to the public health needs when a counterfeit has been reported and has been confirmed. To improve this process, the agency evaluated its policies and procedures for responding to reports of counterfeit drugs to determine if FDA's response could be more efficient. Although FDA has had many positive experiences in responding and working with manufacturers and the public, FDA identified several ways to further enhance coordination and communication among all initial responders within the agency.
Because different parts of the agency throughout the country may receive the
potential counterfeiting report, in some instances, it may take time for the
information to flow to the appropriate people who need it to respond efficiently.
Therefore, FDA has established an FDA-wide rapid response protocol for suspect
counterfeit drugs that will ensure that specified persons/offices/divisions
within the agency are notified and engaged as soon as possible after the report
is made to the agency. Policies and procedures have been or will be amended
to reflect this streamlined information flow and coordination of agency response.
Increased coordination and communication will help FDA to initiate rapidly any
criminal or civil investigation, as well as to assess the health hazard of the
counterfeit situation so the public health response can be launched.
|
To respond rapidly to a report of a suspect counterfeit, FDA is further streamlining its internal processes to respond quickly to reports of suspect counterfeit drugs by improving coordination and communication among all initial responders in the agency.
|
7. EDUCATING THE PUBLIC AND HEALTH PROFESSIONALS
· As the sophistication of the "final product" drug counterfeiting operations has increased, the public needs to be more aware of ways to identify the risk of counterfeit drugs, receive instructions on ways to minimize the chance of receiving fake products and to identify potential counterfeits.
The comments stated that it is imperative that consumers be encouraged to be more proactive in managing their health and be given useful tools to be vigilant to help avoid potential counterfeit drugs. Consumers should be educated to be aware of noticeable differences in their medication, the packaging, or any adverse events. In addition, consumers should understand the important role that their pharmacist and healthcare providers can play in identifying, reporting, and responding to counterfeit drug events. However, the comments warned that care should be taken in any education campaign to not unnecessarily alarm the public.
Despite the growing sophistication of counterfeit drug threats, many consumers are not fully aware of these risks. The Agency, in conjunction with consumer and patient advocates, as well as industry representatives is eager to find additional creative ways to educate the public of the potential threat of counterfeit drugs. The messages should alert consumers to the risk, offer ways consumers can recognize the signs of a potentially counterfeit product, teach them how to reduce the risk of exposure and tell them what to do if they suspect they have encountered one. Of course, FDA wants to strike an appropriate balance in the need to proactively educate consumers without causing unnecessary alarm that could interfere with their use of prescribed drug regimes. Most important, it is critical to focus awareness, and education programs should focus on issues that consumers can control.
FDA has an ongoing educational campaign that is intended to educate consumers about the risks of buying medicines online. FDA intends to reaffirm this message and focus the educational campaign on teaching safe purchasing methods. Particular focus will be placed on encouraging the public to seek out the Verified Internet Pharmacy Practice Site (VIPPS) seal when purchasing from an online pharmacy.
In addition, stakeholders indicated that there is a need for better, timelier, accurate information about specific counterfeit situations. FDA plans to create a counterfeit drug resource page on our website. The objective of this webpage is to concentrate customized education tools into a resource library that can empower individual stakeholder groups.
|
Educating the consumers about the risks of counterfeits is a critical piece in the effort to stop counterfeits from entering the stream of commerce.
|
b. Pharmacists and Other Health Care Professionals
Groups representing pharmacists and pharmacies recognize the need for pharmacists to take a leadership role in the identification of counterfeits, prevention of their introduction into the distribution chain, and education of consumers about counterfeits.
The healthcare community indicated that awareness and education campaigns are important if its health professionals are to be active participants in the fight against counterfeit drugs.
Pharmacists and health professionals can play a major role in helping identify counterfeits and preventing their introduction into the distribution chain. FDA has been working with pharmacy and medical professional groups to develop educational materials for pharmacists and other healthcare professionals, including doctors, nurses, and physician assistants.
|
FDA plans to enhance its educational programs for pharmacists and other heath professionals about their role in minimizing exposure to, identifying, and reporting counterfeits.
|
The comments supported FDA involvement in global efforts to deter and detect counterfeit drugs.
The growing global prevalence of counterfeit drugs must be curtailed. The steps described in this report are intended to secure the U.S. domestic drug supply. However, as long as counterfeit drugs exist worldwide, opportunities could arise for counterfeit drugs to find their way into the U.S. Many countries have taken steps to secure their nation's drugs supply, while others struggle because of limited resources, inadequate regulatory infrastructure, or competing national health priorities. The World Health Organization (WHO) has taken the lead to increase worldwide collaboration and to develop strategies to deter and detect counterfeit drugs. There are several international criminal enforcement collaborations, such as the Permanent Forum on International Pharmaceutical Crime and the Interpol Intellectual Property Crimes Action Group. FDA intends to work with WHO and other international organizations to develop and implement worldwide strategies to combat counterfeit drugs.
|
FDA will collaborate with foreign stakeholders to develop strategies to deter and detect counterfeit drugs globally. |
Below is a table showing when certain anti-counterfeiting measures will be available:

Appendix A: Counterfeit Alert Network Co-sponsorship Agreement
The U.S. Food and Drug Administration (FDA) is committed to informing the public, particularly consumers, pharmacists, other health care professionals, wholesalers, and others involved in the U.S. drug distribution system, about counterfeit drug incidents in a timely manner and educating these parties on ways to identify and prevent counterfeits from entering into this system. To increase awareness of counterfeit drugs and safeguard the nations drug supply, FDA will create a network of national organizations, consumer groups, and industry representatives to deliver time-sensitive messages and information about specific counterfeit incidents and educational messages about counterfeits in general. FDA also will develop and execute informational strategies for specific audiences to ensure that the messages reach the largest number of interested people possible through the network. The network will be called the "Counterfeit Alert Network."
The goals of the Counterfeit Alert Network include, but are not limited to:
This partnership will increase the potential audience of FDA's important notifications about specific counterfeit drug incidents and messages about how and when to report suspect counterfeit drugs. By distributing FDA-developed messages through the [ORGANIZATION] information system, these messages can reach more than [#] people.
FDA will develop targeted messages, with a particular focus on consumers, pharmacists, and other health care professionals when a counterfeit drug is found in the U.S. distribution system. FDA will also develop educational and informational materials about how to detect a counterfeit drug, what to do if a drug is believed to be counterfeit, how to report the suspect counterfeit to the FDA, and ways to minimize the risk of receiving a counterfeit drug. These materials may include: web-based documents, print ads, posters, prepared newspaper articles, fact sheets, consumer brochures/pamphlets, and informational packets. FDA will provide any logistical and technical support, such as writing, layout, designing, and preparing illustrations for the products.
FDA will ensure that all materials are cleared through the Agency and the U.S. Department of Health and Human Services before releasing material to the [ORGANIZATION] for public distribution FDA will provide these materials in a format (hard copy, digital, or electronic) that [ORGANIZATION] can use, as appropriate, to create, manufacture, and/or have printed in enough quantities to distribute to various audiences. FDA will not be responsible for any costs outside of the materials already produced by FDA.
[ORGANIZATION] will distribute in a timely manner FDA's notifications about specific counterfeit incidents as an alert through an active messaging system (separate email or fax alert correspondence). [ORGANIZATION] will facilitate the ability of their members/subscribers/website visitors to report suspect counterfeit drug products to FDA, e.g., via a link to the FDA Counterfeit Drugs webpage or FDA's MedWatch webpage. [ORGANIZATION] will distribute relevant FDA -educational messages about counterfeits, covering such issues as awareness, recognition, prevention, tracking, and authentication of drug products.
The [ORGANIZATION] will pay for the cost, if any, of printing materials, posting materials on its website, email distribution, renting ad space, and securing print placement in magazines and newspapers, as appropriate. [ORGANIZATION] will make clear, in any solicitation for funds to cover its share of the distribution costs that it, not FDA, is asking for the funds. [ORGANIZATION] will not imply that FDA endorses any fundraising activities in connection with the event. [ORANIZATION] will make clear to donors that any gift will go solely toward defraying the expenses of [ORGANIZATION], not FDA.
FDA and the [ORGANIZATION] will develop a dissemination plan that outlines where and how the educational materials and alert messages about specific counterfeit incidents will be distributed to various audiences.
FDA and the [ORGANIZATION] will review this agreement in two (2) years from the original date of this agreement, but either party to this agreement can terminate its participation at any time by notifying the other party of its intent to do so in writing.
The [ORGANIZATION] will not sell any educational materials related to this joint effort. [ORGANIZATION] will not impose an enrollment or registration fee for subscribers to receive this information.
All materials and efforts related to the Counterfeit Alert Network will be jointly sponsored. FDA staff will not be used to develop, promote, or otherwise support any event that is independently sponsored by the co-sponsor, although official announcements and brochures may contain factual references to the available materials and Counterfeit Alert Network messages.
The [ORGANIZATION] will not use the name or logo of FDA except in factual publicity. Factual publicity includes materials provided to [ORGANIZATION] on FDA's program and Counterfeit Alert Network materials. Such factual publicity shall not imply that the involvement of FDA serves as an endorsement of the general policies, activities, or products of the [ORGANIZATION]. Where confusion could result, a disclaimer should accompany publicity to the effect that no endorsement is intended. The [ORGANIZATION] will clear all publicity materials with FDA to ensure compliance.
Records concerning this partnership shall account fully and accurately for any financial commitments and expenditures of FDA and [ORGANIZATION]. Such records shall reflect, at a minimum, the amounts, sources, and uses of all funds.
This co-sponsorship agreement, as well as any financial records for this partnership, shall be publicly available.
FDA and the [ORGANIZATION] will abide by the memorandum of August 8, 2002, "Co-sponsorship Guidance," issued by the Associate General Counsel for Ethics.
__________________________ ___________________
DATE
FDA Signee
_________________________ ___________________
[NAME] DATE
[TITLE]
[ORGANIZATION]
__________________________ ___________________
Director, Ethics and Integrity Staff DATE
Office of Management and Programs
Office of Management
Food and Drug Administration
Unit of Use Packaging
Comments supporting widespread utilization of unit of use technology cited:
Some comments cautioned the FDA against mandating unit of use packaging for all drugs citing:
Authentication Technologies
They supported use of authentication technologies as part of an overall anti-counterfeiting strategy and stated that authentication technologies serve two purposes:
Manufacturers of specific anti-counterfeiting technologies provided us with descriptions of their products that were extremely valuable in helping us understand how they work, their cost, and how they might be incorporated into pharmaceutical products, packaging, and labeling or used to detect counterfeit products through forensic and other analytical methods, including rapid methods.
Many comments supported the issuance of an FDA guidance document on the use of authentication technologies. They stated that there was no clear FDA policy specifically targeted to this important subject. They suggested that current FDA policies and practices for New Drug Applications (NDAs), Abbreviated New Drug Applications (ANDAs), and Biologics License Applications (BLAs), supplements, and other notification procedures should be clarified so the policies and procedures applicable to use of anti-counterfeiting technologies are clearly articulated and available in a single document.
The following points were made regarding the use of authentication technologies on drug products, their packaging and labeling:
List of Drugs Likely to be Counterfeited
Many comments stated that it was important for stakeholders to allocate financial resources to protect those products that are most likely to be counterfeited.
There was agreement that the criteria we suggested to identify drugs that were likely to be counterfeited were correct. These included:
However, there was no consensus on how to apply these, or other, criteria in creating a list of such products.
As stated above, some comments suggested that instead of developing a list of drugs likely to be counterfeited, a set of criteria for determining whether a drug was at likely to be counterfeited should be created. One proposal for such criteria was:
A drug has been subjected to a seizure or stop sale notice because of counterfeiting,
or
There is documentation that a drug was counterfeited and is the subject of an
investigation by federal or state authorities
AND
The product is high cost (e.g., over $200 per dose) or high volume (e.g., top
fifty drugs), or
The product is used extensively for treatment of HIV/AIDS or cancer, or
The product is injectable, or
The product distributed in a special or limited way, or
There are multiple documented instances of pedigrees not being passed with the
product
Radiofrequency Identification Technology
We received a large amount of information on the benefits, costs, and unresolved issues relating to RFID. These include:
Benefits
Costs
Unresolved Issues
Stakeholder Activities
We have been informed of several feasibility studies, starting in early 2004, that should give members of the supply chain experience using RFID as well as provide them with an opportunity to test its business uses and identify potential barriers to its acceptance. These studies include:
In addition to feasibility studies, we understand that several groups representing many supply chain participants have been meeting to discuss ways to facilitate the adoption of RFID. For example the Product Safety Task Force (PSTF) convened under the auspices of the Healthcare Distribution Management Association (HDMA) is developing business requirements and identifying business issues relating to RFID technology.
The PSTF and other stakeholders have informed us that the migratory path (or phase in) to widespread use of RFID at a package level could vary by stakeholder based on the place of that stakeholder in the supply chain (e.g., manufacturer vs. retailer) and on specific costs and benefits accruing to that stakeholder (e.g., types of products manufactured, number of distribution centers, technology cost per product).
Several migratory paths were mentioned, including:
According to stakeholders, these paths are not mutually exclusive and it is likely all of these, and other, paths will be utilized as RFID technology becomes more widely adopted.
Below are some of the secure business practices that have been developed by participants in the U. S. drug distribution system.
Several manufacturers have announced policies intended to secure the supply
chain. These policies include:
The Healthcare Distribution Management Association (HDMA) released a document entitled "Recommended Guidelines for Pharmaceutical Distribution System Integrity" which set forth a series of recommended actions for wholesalers to take prior to and while conducting business transactions with other wholesalers. In essence they comprise a "due diligence" checklist which includes items such as:
Individual wholesalers supported the HDMA guidelines and provided FDA with ideas for additional secure business practices including:
We have been informed that several organizations representing pharmacies and pharmacists are developing secure business practices as a guide for pharmacies and pharmacists. One pharmacy group notified us that they have already published a list of strategies to use for assuring the integrity of pharmaceuticals. This list includes:
A majority of the comments that discussed PDMA noted the limitations and concerns of full implementation of PDMA. Such limitations include:
A number of other comments, however, supported the use of paper pedigrees for their deterrent value and as a means to verify prior sales through due diligence. Comments noted that even forged pedigree papers provide an additional opportunity to identify counterfeiters and block introduction of counterfeit drugs into the drug supply if wholesalers exercise due diligence by tracing the sales through the pedigree and identifying the place where the forgery occurred. A few comments suggested that FDA should exercise enforcement discretion and not take enforcement action against a wholesaler who fails to provide pedigree information back to the manufacturer as long as the wholesaler provides pedigree information back to the first ADR who received the drug from the manufacturer.
Several comments suggested a risk-based approach to implementation of the PDMA,
which focuses on those drugs that are at high-risk of being counterfeited. Many
of these comments suggested that high-risk drugs maintain a full pedigree that
documents all sales and transactions back to the manufacturer. One comment suggested
an interim solution of "one forward, one back" pedigree for high-risk
drugs. This system would be analogous to recent bioterrorism legislation for
food distributors, whereby participants in the food distribution system maintain
only those records necessary to identify immediate previous sources and immediate
subsequent recipients of food. However, comments on FDA's food regulations
have suggested it will take at least several years to phase in the paper recordkeeping
requirements. Moreover, in contrast to drugs, there are no major steps in development
now to provide widespread electronic pedigrees for drug products. Finally, as
noted throughout the riskiest drug products are the ones for which modern anti-counterfeiting
and track-and-trace methods should be implemented soonest.
Most comments supported the development of an electronic pedigree for all drug
products in the supply chain and that an electronic pedigree should be considered
as a long-term solution to fulfilling the PDMA requirements codified at 21 CFR
203.50. Given the costs of implementing the partial anti-counterfeiting measures
included in the PDMA, and the expectation of continued significant progress
toward implementation of modern pedigree systems for drugs, more effective modern
pedigree systems are likely to be available before it would be possible to phase
in and achieve compliance with paper pedigree requirements.
The comments overwhelmingly supported strengthening requirements governing the licensure and oversight of wholesale distributors. Many comments cited the systemic weaknesses in the oversight of the wholesale drug industry, prior to Florida's implementation of licensing reform, that were described in the Florida Grand Jury Report, such as issuing licenses without proper background checks and granting licenses despite one or more felony convictions. The comments also stated that existing inspection and due diligence processes are often insufficient to detect criminal activity. As mentioned above, there was uniform agreement that the penalties for counterfeiting drugs are insufficient to serve as an adequate deterrent.
Many comments supported the concept of tighter requirements generally, while others gave specific suggestions for improvement. Some of the specific suggestions included:
Most comments stated that the stricter standards should be uniform across all
50 states so as not to create 50 different sets of criteria and rules for licensing.
Concerns about several provisions in the new Florida and Nevada laws regarding
licensing of wholesale distributors were expressed. Some of the comments described
implementation and logistical problems that wholesalers have experienced in
these states as a result of the new law.
Some comments encouraged FDA to revisit the minimum standards requirements described in 21 CFR Part 205 to create a 'federal floor' for States to meet. The comments were not uniform, however, on whether such a federal floor might enhance or deter state efforts to implement the complete set of NABP recommendations.
The agency received many supportive comments about the counterfeit alert network
concept. Most of the comments suggested that the agency use existing networks
and several comments offered their organizations distribution list or network
as a conduit for the counterfeit alert network.
Some comments offered strategic approaches for the development of such a network,
including suggested concepts for message delivery. Suggestions include using
active notification via "push" email technology, validated and secure systems,
easily understood language with clear and unambiguous messages, multiple notification
systems, accessible to all stakeholders, no cost for users, timely, visual alert
to flag importance, redundant delivery vehicles such as email, fax, direct mail,
and phone, and have an embedded link to take user back to FDA or MedWatch website.
The comments also suggested that consistency is an important element so there
is familiarity in times of emergency situations. The agency was warned not to
overuse the counterfeit alert network in order to avoid 'alert fatigue,' which
could create indifference or doubt regarding the importance of the messages.
The agency was encouraged to consider public/private partnerships to design
communication strategies and facilitate efforts to standardize anti-counterfeit
communications and to augment and coordinate communication systems. The comments
also said that costs to FDA and private partners should be kept to a minimum.
![]()
1The Task Force consists of senior agency staff from the Office of the Commissioner (Office of Policy and Planning, Office of External Affairs, and Office of the Chief Counsel), Office of Regulatory Affairs, the Center for Drug Evaluation and Research, and the Center for Biologics Evaluation and Research.
![]()