Publications:
Pentacel Guidance
![]() Print-friendly version of this page
Print-friendly version of this page ![]() (31 KB)
(31 KB)
August 2008
| Pentacel is a combination vaccine that contains DTaP, IPV and Hib vaccines. Pentacel is supplied as single-dose vials, 5 doses to a package. A single-dose vial of liquid DTaP-IPV vaccine is used to reconstitute a single-dose vial of lyophilized ActHIB vaccine. The vaccine must be kept at refrigerator temperature (35° -46° F) at all times. Pentacel must never be frozen. Vaccine exposed to freezing temperature must not be used. | 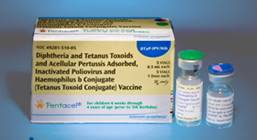 |
IMPORTANT NOTE: The availability of Pentacel will improve the Hib vaccine supply situation in the United States. However, the availability of Pentacel is not sufficient to reinstate the last (booster) dose of the Hib vaccine series (i.e., the dose administered after the first birthday). Although Pentacel is licensed by FDA for the fourth dose in the DTaP, IPV and Hib series, providers should NOT use it for the fourth dose until there is further improvement in the Hib vaccine supply (anticipated for the last quarter of 2008). Until the Hib supply improves Pentacel should be used ONLY for the first three doses of the DTaP, IPV, and Hib vaccination series, except as noted below.
As with all combination vaccines, there are no special rules for Pentacel, except as determined by FDA licensure of the product (i.e., the maximum age for any dose—see below). The schedule, minimum intervals, and minimum ages are determined by the individual components. The recommended schedule for Pentacel is similar to those for DTaP and ActHib with doses at 2, 4, 6, and 15 through 18 months of age.
Pentacel can be administered to any child 6 weeks through 4 years of age, without a contraindication to any component, for whom DTaP, IPV, and Hib vaccines are indicated. As stated on the childhood immunization schedule, a combination vaccine, including Pentacel, may be used whenever any component(s) of the combination is indicated and no other component of the vaccine is contraindicated. This means that Pentacel can be used when a child needs one or two components, but does not need the others.
Contraindications and precautions for Pentacel are the same as those for DTaP, IPV, and Hib vaccines.
The following minimum ages and intervals are defined for the component vaccines in various ACIP statements, and in particular in Table 1 of the 2006 version of the General Recommendations on Immunization (http://www.cdc.gov/mmwr/PDF/rr/rr5515.pdf, page 3) and on page 31-32 of the 2006 AAP Red Book.
Parameter |
Age/interval |
|---|---|
Minimum age for any dose |
6 weeks |
Minimum interval for doses 1 and 2 |
4 weeks |
Minimum age for dose 2 |
10 weeks |
Minimum interval for doses 2 and 3 |
4 weeks |
Minimum age for dose 3 |
14 weeks |
Minimum interval for dose 3 and 4 |
6 months (determined by DTaP component; minimum interval for dose 3-4 is two months for Hib and four weeks for IPV) |
Minimum age for dose 4 |
12 months (determined by DTaP and Hib components). Note that both the minimum interval AND age must be met for the fourth dose of DTaP or Hib (as Pentacel or any other formulation) to be counted as valid |
Maximum age for any dose |
4 years, 364 days (i.e., do not administer at age 5 years or older) |
Please refer to the examples below for guidance on schedules for Pentacel, Pediarix and the single antigen series for Hep B, Hib, IPV DTaP for healthy children* during the Hib vaccine shortage.
Examples of Schedules Using Pentacel and/or Pediarix for Healthy Children* During the Hib Shortage
The first two examples below provide instances of how to introduce Pentacel in your practice using two different schedules. The second two examples review the schedules for the single antigen and Pediarix series for Hep B, IPV, Hib and DTaP.
Schedule for Hep B, Hib*, IPV, and DTaP Using Pentacel for All Doses
Birth: Hep B
2 months: Hep B and Pentacel
4 months: Pentacel
6 months: Hep B and Pentacel
15-18 months: DTaP
4-6 years: DTaP and IPV
Schedule for Hep B, Hib*, IPV, and DTaP Using Pentacel For First Dose Only
and Pediarix for Remainder of Doses
Birth: Hep B
2 months: Hep B and Pentacel
4 months: Hib and Pediarix
6 months: Hib and Pediarix
15-18 months: DTaP
4-6 years: DTaP and IPV
Schedule for Hep B, Hib*, DTaP and IPV Without Pentacel or Pediarix
Birth: Hep B
2 months: Hep B, Hib, DTaP, and IPV
4 months: Hib, DTaP, and IPV
6 months: Hep B, Hib, DTaP, and IPV
15-18 months: DTaP
4-6 years: DTaP and IPV
Schedule for Hep B, Hib*, IPV, and DTaP Using Pediarix Only (No Pentacel)
Birth: Hep B
2 months: Hib and Pediarix
4 months: Hib and Pediarix
6 months: Hib and Pediarix
15-18 months: DTaP
4-6 years: DTaP and IPV
Pentacel contains DTaP, IPV and Hib. Pediarix contains DTaP, IPV, and Hep B
Neither Pentacel nor Pediarix should be used prior to 6 weeks of age. In general ACIP recommends the same brand of DTaP be used for all doses of the series. However, different brands can be used if the provider does not know or have available the brand of DTaP used for prior doses.
*When supplies are sufficient an additional dose of Hib vaccine (single antigen or as part of a combination vaccine) is recommended for healthy children at 12 through 15 months of age (at least 2 months after the prior dose). Either Pentacel or single antigen Hib vaccine may be used at 12 through 15 months of age for children who are at increased risk of Hib disease or who have not completed a complete primary Hib schedule. If Pentacel is administered at 12 through 15 months of age a dose of DTaP at 15 through 18 months of age is not needed. See MMWR 2007;56(No.50):1318-1320 for additional details.
Questions or comments on this document should be directed to your state or local immunization program, or to CDC by e-mail at nipinfo@cdc.gov.
Copyrighted images: Images on this website which are copyrighted were used with permission of the copyright holder and are not in the public domain. CDC has licensed these images for use in the materials provided on this website, and the materials in the form presented on this website may be used without seeking further permission. Any other use of copyrighted images requires permission from the copyright holder.
.pdf files: To view and print the .pdf files on this site, you will need Adobe Acrobat Reader. Use this link to obtain a free copy of Adobe Acrobat Reader (exit). We highly recommend that you upgrade to the latest version if haven't already.
Content last reviewed on August 11, 2008
Content Source: National Center for Immunization and Respiratory Diseases
