The news media report advances in genetic research almost every day. Recent discoveries have associated specific gene variants with the development of disease or chronic conditions, many of which affect broad segments of the population. For example, BRCA1 is associated with a high risk for breast and ovarian cancer, CCR5 confers protection against HIV infection and the development of AIDS, and hereditary hemochromatosis leads to significant morbidity and mortality from iron overload. Simultaneous with these advances, genetic tests are increasingly being developed and made publicly available.
Putting this information to good use to promote the health and well-being of all members of society requires a keen understanding of complex issues. Chief among these issues are the ethical ramifications of using new genetic technologies, and variations in personal and cultural views on what constitutes disease and disability. Another issue concerns the accumulation of epidemiologic information on how results from genetic testing can be used to prevent disease manifestation. The interaction between genotype and environmental factors--such as behaviors and exposures--also calls for extensive study to determine how such factors can be modified to improve health outcomes. The value of certain genetic tests in disease prevention and health promotion efforts has yet to be determined, and standards for laboratory testing are only evolving. Policies are needed to ensure the appropriate use of predictive genetic testing and counseling and to prevent inappropriate use of such testing, particularly for diseases (such as Huntington's disease and Alzheimer's disease) for which effective environmental, behavioral, or medical interventions are lacking. People must be assured that information about their genetic composition will remain confidential and that they will receive appropriate counseling about treatment options for known conditions.
These issues illustrate the multifaceted public health dimensions of genetic research, which is already changing the practice of medicine and will have a profound effect on health care in the new millennium. CDC, in concert with other federal agencies and in collaboration with many public and private partners, can assist public health professionals in promoting health and preventing disease and disability among people for whom the consequences of an inherited risk can be ameliorated. The recommendations in this strategic plan will help ensure that results from genetic research are responsibly used in public health practice. Each of these recommendations has potential policy, coordination, partnership, and resource implications.
By the year 2005, most--if not all--of the estimated 100,000 human genes will have been identified, and tests for more than 400 genes are already available in medical practice. Genes found thus far include not only those associated with rare diseases, but also genes that increase susceptibility to common diseases. Risk for disease increases when genes interact with environmental factors, including chemical, physical, and infectious agents, and behavioral or nutritional factors.
How to use knowledge from genetics research to promote health and prevent disease and disability is now being explored. But information is lacking about the benefits and risks of genetic testing, the efficacy of early interventions, and the population distribution of genotypes and other risk factors associated with disease conditions. Moreover, the complex and controversial issues that have emerged--such as the quality of laboratory testing, the rapid commercialization of genetic tests, the availability of and people's access to interventions, and the potential discrimination against and stigmatization of individuals and groups--call for public health leadership.
As the nation's prevention agency, CDC must collaborate with other federal agencies and many partners in ensuring that advances in medical genetics are appropriately used for promoting health and preventing disease and disability. In September 1996, CDC's Director, Dr. David Satcher, appointed the agency-wide, ad hoc Task Force on Genetics in Disease Prevention to propose a strategic plan through which the agency might coordinate and strengthen its activities in genetics and public health. Specifically, the primary functions of the task force were to (1) develop a strategic plan for CDC-wide genetics activities, (2) coordinate and support efforts involving multiple programs at CDC, and (3) convene constituents and consultants to obtain their advice on strategic planning and priorities for CDC activities related to genetics in public health.
This strategic plan focuses on human genetics only and is based on the assumption that the use of genetic information in public health is appropriate in diagnosing, treating and preventing disease, disability, and death among people who inherit specific genotypes. Prevention includes the use of medical, behavioral, and environmental interventions to reduce the risk for disease among people susceptible because of their genetic makeup and does not include preventing the of birth of people with specific genotypes. This plan supports the responsible use of genetic tests and services, including adequate family history assessment and genetic counseling, for promoting health and preventing disease in different communities. The plan assumes that the delivery of genetic tests and services will be done within the context of the evolving health care system, including managed care organizations, rather than by public health agencies. Public health agencies will, however, have an increasing role in assessing the health needs of populations, assuring the quality of genetic tests and services, and evaluating the impact of interventions. This plan does not discuss clinical practice guidelines for individual patients and their families.
The task force developed a conceptual framework for a public health program in genetics. The framework identifies four essential program components--public health assessment; evaluation of genetic testing; intervention development, implementation, and evaluation; and communication and information dissemination--and three critical issues that affect each component--partnerships and coordination; ethical, legal, and social issues; and education and training.
Using this framework, the task force developed seven broad goals that collectively form a strategy for strengthening CDC's existing activities and developing new initiatives in genetics and public health. These long-term goals can be achieved through the coordinated efforts of the agencies of the Department of Health and Human Services (HHS), in collaboration with state and local health departments, schools of public health, and professional, academic, industry, and consumer organizations.
- Goal 1: Foster partnerships and coordination of genetic activities within and outside of CDC to promote health and prevent disease and disability.
- Goal 2: Ensure that ethical, legal, and social issues are addressed in applying genetics to the promotion of health and the prevention of disease and disability.
- Goal 3: Assess how risk for disease and disability is influenced by the interaction of human genetic variation with modifiable risk factors.
- Goal 4: Ensure the appropriateness and quality of population-based genetic testing.
- Goal 5: Ensure that genetic tests and services are incorporated into population-based interventions that promote health and prevent disease and disability.
- Goal 6: Build capacity to promote health and prevent disease and disability by training public health professionals in genetics.
- Goal 7: Provide timely and accurate information to both the general public and professional audiences on the role of genetics in the promotion of health and the prevention of disease and disability.
After developing supporting objectives, the task force recommended specific steps that CDC can take to fulfill these goals. The recommendations for immediate action are listed below. Each of these recommendations has potential policy, coordination, partnership, and resource implications. The implementation of each recommendation will require the availability of adequate resources.
Sustain a coordinated focus on genetics and public health at CDC.
The program activities recommended in this strategic plan will be implemented by CDC's various centers, institutes, and offices. A CDC
National Office of Public Health Genomics should perform the following functions: (1) coordinate genetics activities throughout CDC and ensure that these activities are consistent with the policies and priorities of the Department of Health and Human Services; (2) develop guidelines for setting cross-cutting priorities in public health genetics; (3) assess needs for professional expertise and capacity for activities in genetics at CDC and at the state level; (4) cultivate partnerships with external agencies, organizations, and constituencies to coordinate efforts and ensure a productive exchange of information and concerns; (5) assess how current and proposed laws, regulations, and rulings affect genetics activities at CDC and the state level (in collaboration with CDC's Office of the Director); and (6) coordinate workshops on critical cross-cutting issues (e.g., informed consent), from which guidelines and recommendations may emerge.
Establish mechanisms for external input, particularly for ethical, legal, and social issues.
Create a liaison with the Ethical, Legal, and Social Implications program of the National Institutes of Health's National Human Genome Research Institute. Consider establishing a genetics subcommittee of the CDC Advisory Committee and ensure that these efforts are coordinated with other HHS advisory committees on genetics. Convene external consultants from different organizations to discuss early implementation steps of this strategic plan and seek regular external input thereafter. These mechanisms can help ensure that CDC remains connected with and responsive to a broad community that can help anticipate and address the complex philosophical and practical issues of genetics at CDC.
Provide training opportunities to enhance the skills and knowledge of public health professionals.
Develop an introductory training course in genetics and public health that includes a module on ethical, legal, and social issues. Sponsor a CDC-wide career development program to develop additional expertise in genetics in public health. Develop a network of professionals involved in epidemiologic studies of human genes to foster collaboration, information sharing and training. These educational activities will help increase understanding regarding the role of genetics in disease prevention and fill gaps in professional knowledge.
Develop a strategy for communication about genetics.
In collaboration with CDC's Office of Communication, conduct a comprehensive review of communication research in genetics, develop a plan for assessing the information needs of various audiences, develop messages, and select media for disseminating information about genetics and public health. Use the Internet as one distribution mechanism. These activities will ensure that the dissemination of information is coordinated, accurate, and timely.
Sponsor intramural activities on applied genetic research.
Make seed money available, through a competitive funding process, to support startup genetics projects throughout CDC. Available funding from CDC can be supplemented with funds from the different centers, institutes, and offices. Establish a panel for the objective review and ranking of proposals according to agreed upon criteria. These projects will allow CDC to expand ongoing activities and build scientific credibility and capacity for new activities in multiple centers, institutes, and offices.
Expand activities to ensure the quality of genetic testing.
Establish a genetics subcommittee for the advisory committee of CLIA (Clinical Laboratory Improvement Act Amendments of 1988). In collaboration with the Food and Drug Administration and the Health Care Financing Administration, improve CLIA regulations by creating a new category for molecular diagnostics. Collaborate with professional organizations such as the American College of Medical Genetics and the College of American Pathologists to define CDC's role in relation to other organizations in quality assurance for genetic testing. Conduct studies on methods to improve testing performance and to develop guidelines and models for quality assurance.
Sponsor extramural projects to evaluate intervention programs that use genetic tests and services.
Use a competitive process to evaluate ongoing interventions (e.g., sickle cell disease, familial hypercholesterolemia) and assess prevention effectiveness. Support for extramural activities should focus on applied research in diverse populations and various settings. The amount of funding will depend on availability of resources.
Sponsor the first annual meeting on genetics in public health.
Create a forum for the ongoing exchange of information on the application of genetic advances to public health practice. Sessions can cover recent discoveries, ethical concerns, data sources, programmatic considerations, policy issues, information technology, and other topics. Professionals from various disciplines can share experiences and concerns about the impact of genetic advances on public health.
Translating Advances in Human Genetics into
Public Health Action: A Strategic Plan
Advances in genetic technology
In 1990, the Human Genome Project was jointly started by the National Institutes of Health (NIH) and the Department of Energy (DOE) (Watson, 1990; Guyer and Collins, 1995). By the year 2005, most--if not all--of the estimated 100,000 human genes will have been found. More than 8,000 genes have already been cataloged (Online Mendelian Inheritance in Man [OMIM], 1997), and tests for more than 400 genes are available in medical practice.
The genes identified thus far include not only those associated with rare metabolic disorders and specific malformation syndromes, but also genes that increase susceptibility to common diseases. When such susceptibility genes interact with the environment (which includes chemical, infectious, physical, social, psychological, behavioral, and nutritional factors), the risk is increased for major chronic diseases, such as cancer, cardiovascular disease, and Alzheimer's disease.
|
Number of Genes* Reported in OMIM for Selected Conditions |
| |
|
Condition |
Number of Entries |
| Mental Retardation |
802 |
| Inborn Errors of Metabolism |
518 |
| Congenital Anomalies |
492 |
| Cancer |
367 |
| Anemia |
288 |
| Infection |
258 |
| Diabetes |
242 |
| Thyroid Disorders |
179 |
| Dementia |
126 |
| Arthritis |
96 |
| Myocardial Infarction |
44 |
| |
|
| *Includes entries for identified or mapped genes. Source: Online Mendelian Inheritance in Man, 1997. |
|
As a result of genetic research, information on people's genetic susceptibility to and risk for disease is accumulating, and complex ethical, legal, and social issues are being raised (Hoffman, 1994; Whelan, 1995; Juengst, 1995). The effective use of this knowledge and technology has become a burgeoning challenge to the public health community (see appendices A-C; Feder et al., 1996; Miki et al., 1994; Dean et al., 1996).
Yet the use of this knowledge in promoting health and preventing disease and disability has scarcely been explored. Information is lacking about the population distribution of genotypes and other risk factors associated with disease conditions, the benefits and risks of genetic testing, and the efficacy of early interventions. The complex and controversial issues that have emerged--about quality assurance for laboratory testing, rapid commercialization of genetic tests, availability of and access to effective and acceptable interventions, and potential discrimination against and stigmatization of individuals and groups--call for public health leadership.
Need for public health leadership in genetics
As the nation's prevention agency, CDC must collaborate with many partners in ensuring that medical genetics is appropriately incorporated into public health practice (Satcher, 1996). This endeavor falls within CDC's mission (see box) and the core functions of public health agencies: assessment, policy development, assurance, and evaluation (Institute of Medicine, 1988; Khoury and Genetics Working Group, 1996; Omenn, 1996). CDC must coordinate its genetics activities with those of other federal agencies, including but not limited to the National Institutes of Health, the Health Resources and Services Administration, the Agency for Health Care Policy Research, the Food and Drug Administration, and the Health Care Financing Administration.
More than half the centers, institutes, and offices (CIOs) at CDC are conducting research or intervention activities in genetics and public health (see Appendix D). Some of these activities are well established and nationally recognized, and some are still evolving. These activities indicate not only the breadth and depth of activity in genetics at CDC, but also the considerable expertise in genetics and public health throughout the agency.
The mission of CDC, an agency of the Department of Health and Human Services, is to promote health and quality of life by preventing and controlling disease, injury, and disability. CDC accomplishes its mission by collaborating with other federal agencies, states, and the private sector in detecting and investigating health problems, conducting population-based surveillance, and developing and evaluating prevention programs.
|
|
Strategic planning or genetics in public health
In September 1996, Dr. David Satcher, CDC's Director, appointed the agency-wide, ad hoc Task Force on Genetics in Disease Prevention to propose a strategic plan through which the agency might coordinate and strengthen its activities in genetics and public health. Specifically, the primary functions of the task force were to (1) develop a strategic plan for CDC-wide genetics activities, (2) coordinate and support efforts involving multiple CIOs at CDC, and (3) convene constituents and consultants to obtain their advice on strategic planning and priorities for CDC activities related to genetics in public health. The main objectives of the task force were to identify CDC's prevention activities in genetics, evaluate current practices, identify the issues of greatest concern in public health and genetics, and outline CDC's future role in genetics.
The task force, which included people with diverse backgrounds from throughout CDC (see Appendix E), solicited input from other CDC staff members and from external sources, including people from academia, professional organizations, consumer groups, state health departments, and federal agencies. After its inception, the task force met weekly and held several retreats to debate the implications of multifaceted issues; conducted a survey to identify genetics-related activities and possible future efforts at CDC; held a two-day meeting of outside consultants to discuss CDC's future role in genetics and public health (see appendices F and G); and drafted this strategic plan. Many of these activities, including consultation with representatives of outside agencies, groups, and organizations, are ongoing. The task force also received support and guidance from an oversight group created by Dr. Satcher. The oversight group, which consisted of representatives from the Office of the Director and each CIO, is co-chaired by Dr. James Marks (Director, National Center for Chronic Disease Prevention and Health Promotion) and Dr. Richard Jackson (Director, National Center for CDC, helped Environmental Health). The oversight group provided input on policy and programmatic implications for CDC, helped ensure that programs throughout CDC were informed about the actions of the task force, and reviewed the strategic plan developed by the task force.
The task force was aware of the important efforts of several other groups making recommendations on genetic issues, such as the National Bioethics Advisory Commission, and the committee chaired by Dr. Mark Rothstein that reviewed the Ethical, Legal and Social Implications Program at the National Institutes of Health. In particular, the task force acknowledges the important contributions of the joint NIH-DOE Task Force on Genetic Testing (TFGT), chaired by Dr. Neil Holtzman, which had representatives from various private, professional, industry, and consumer organizations, in addition to representatives from various HHS agencies. The TFGT, which was charged with drafting recommendations to ensure the safety and effectiveness of genetic testing in the United States, produced its final report in May 1997 (see appendix H for selected recommendations). The CDC task force has incorporated many of the recommendations of the TFGT in its deliberations, specifically, those that call upon CDC to play an essential role in gathering population-based data on genetic tests, to expand its surveillance capabilities on the natural history of genetic disorders such as trends in morbidity, disability and mortality associated with various genetic conditions, to conduct epidemiologic studies to learn more about the validity, safety, and effectiveness of genetic tests, and to apply knowledge gained to help ensure a high level of quality in testing.
Philosophical foundation
The development of this plan was guided by the following vision, mission, and value statements drafted by the task force:
Healthier lives through the responsible use of genetic knowledge.
To integrate knowledge of human genetics into effective and ethical public health actions that promote health and prevent disease and disability.
We value the use of the highest quality science as the foundation
for public health policies and practices involving genetics.
We value the health and quality of life of present and future generations.
We value an individual's right to make informed
choices about genetic tests and services.
We value awareness of and access to quality genetic tests
and services, including genetic counseling.
We value the collaborative efforts and contributions of our partners
and stakeholders, both nationally and globally.
We value diversity among people and the uniqueness of the individual.
We value respect for privacy, confidentiality, and the human
rights of individuals and their families.
We value a public health approach that balances the interests of
individuals with the interests of the population as a whole.
|
|
Scope and assumptions
The plan focuses on human genetics only. Other research in genetics that affects public health, such as genetic variation in microorganisms, insect vectors, and other nonhuman species, that affects public health, was considered beyond the scope of this plan.
The plan is based on the assumption that the use of genetic information in public health is appropriate in promoting health and in diagnosing, treating, and preventing disease, disability, and death among people who inherit specific genotypes. Such prevention concerns the use of medical, behavioral, and environmental interventions to reduce the risk for disease among people susceptible because of their genetic makeup. It does not include efforts to prevent the birth of infants with specific genotypes.
The plan is also based on the assumption that most diseases of significant public health impact result from the interaction between genotype and environmental factors. This interaction occurs for rare conditions usually associated with a few genes, as well as for more common diseases associated with multiple genes and environmental factors. Because this interaction occurs, genetic risk factors should be incorporated into the traditional epidemiologic paradigm (Khoury et al., 1993). The paradigm could also account for how gene-environment interaction can increase the risk for various diseases, such as cancer.
The plan assumes that much of the delivery of genetic tests and services for disease prevention and health promotion, including adequate family history assessment and genetic counseling, will be done within the context of the evolving health care system. Managed care organizations will play an important role in integrating genetic services into disease prevention and health promotion activities. Large-scale mandated public health programs are not viewed as the foundation for the implementation of this plan.
As outlined by the Institute of Medicine Report on the Future of Public Health (IOM, 1988), public health agencies will have an increasing role in assessing the health needs of populations, working with the private sector in ensuring the quality of genetic tests and services, and evaluating the impact of interventions on medical, behavioral, and psychosocial outcomes.
Human genetics is a global rather than domestic issue. However, most of the activities proposed in this plan pertain to the U.S. population and involve partnerships at the federal, state, and local levels. CDC's responsibility as a partner in global health should be continually addressed as the strategic plan is implemented.
The task force developed a conceptual framework for a public health program in genetics (Figure 1). The framework identifies four essential program components and three critical issues that affect each component. Although not specifically identified, sound policy development and implementation is inherent in the framework of public health function (Institute of Medicine, 1988).
By program, the task force means a plan through which actions can be systematically taken toward achieving specified goals. For actions to be systematic, certain information necessary for setting priorities must be gathered. This information includes measuring the magnitude of a disease condition associated with a given genotype, the extent to which the condition can be prevented, the risks and benefits of interventions, and the cost of interventions and alternate strategies.
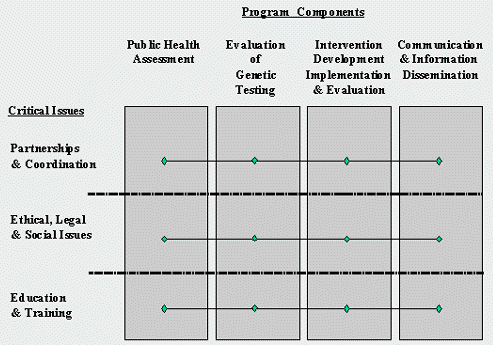
Figure 1: Conceptual Framework for a public health program in genetics
Component 1: Public health assessment
Public health assessment in genetics relies on the use of the highest-quality science in conducting surveillance and epidemiologic studies, the traditional forms of applied research in public health.
Surveillance
Surveillance is needed to determine the population frequency of:
- Genetic variants that predispose people to specific diseases, both common and rare.
- Morbidity and mortality associated with such diseases.
- Environmental factors known to interact with given genotypes in disease-producing ways.
Broadened surveillance would also collect information on the economic costs of genetic diseases (as expressed by health care costs, hospitalization rates, number of work days lost, years of potential life lost, and other measures), issues related to genetic testing (including access to services, quality of tests, usage by providers, and potential discrimination), and issues related to interventions (including availability and effectiveness).
CDC can build on existing surveillance systems and health information systems, such as the National Health and Nutrition Examination Survey (see box), that are maintained by federal agencies, state health departments, and other organizations. However, new models may be needed for surveillance of populations predisposed to a given genetic disease or having high exposure to compromising environmental factors. Further, the limitations of existing systems, such as incomplete coverage, remain concerns for those conducting surveillance in genetics and disease prevention.
-
The DNA Bank contains about 17,000 DNA specimens from a representative sample of the U.S. population. Information about these specimens could be used with other NHANES data to study genetic risk factors for common diseases, such as heart diseases and cancer.
-
About half the specimens are in the form of immortalized cells, which provide an unlimited DNA source for the sample.
-
By offering leadership in addressing the ethical, legal, and social implications of testing these samples, CDC is working to ensure that information is responsibly used.
-
A follow-up study of NHANES III participants may be conducted
|
|
Epidemiologic studies
Population-based studies are needed to identify the environmental factors, such as occupational exposures, diet, and behaviors, that contribute to the development of clinical disease among people susceptible because of their genotype. Such studies can also identify environmental factors that produce genetic alterations that lead to disease. Advances in human genetics may result in improvements in the predictive value of traditional epidemiologic risk factors. Epidemiologic studies that account for genotype or that correlate genotype with clinical findings may also reveal ameliorating factors, new interventions, or ways of applying standard interventions.
Component 2: Evaluation of genetic testing
This component merges two main sets of activities: (1) the assessment of how and when genetic tests are or can be used to promote health and to diagnose and prevent human disease and (2) the development of standards and guidelines for quality genetic testing. As used here, genetic testing refers to analyses (using molecular, biochemical, cytogenic, or other laboratory methods) of biologic specimens to identify genotypes that may influence a person's risk for disease or disability. The task force recognizes that genetic testing should occur in the context of genetic service delivery, including adequate family history assessment and genetic counseling. Although the focus of this program component is the evaluation of genetic testing, it may also include the development of new genetic tests that are suitable for population-based testing. Such developments, however, will not be a major component of a public health genetics program.
Assessment of tests
The first set of activities includes the ongoing review and cataloging of genetic tests. Although activities are needed to evaluate the appropriateness of genetic testing on a population basis and the usefulness of such testing in promoting public health, efforts are also needed to ensure that high-quality genetic testing is available to all population segments, including disadvantaged populations. Availability can be affected by how convenient, affordable, and appropriate genetic testing is for people of specific cultures. By working with prevention partners, CDC can identify gaps in the availability of genetics tests, help develop the infrastructure necessary to increase their availability, and assess changes in their use.
For all populations, the appropriateness of genetic testing can be evaluated in many ways. For example, surveillance of health care providers might reveal which tests are ordered, which people are tested, whether the test is available to all people likely to benefit, and whether interventions are available and correctly applied once test results are known. Additional concerns about evaluation include the cost, sensitivity, and specificity of tests; the methods used to ensure confidentiality of results; and the availability and use of trained personnel for conducting tests and counseling clients about test results. Additional surveillance might seek to assess public awareness, understanding, and use of genetic tests--assessments that might be accomplished by adding questions to long-standing systems, such as the Behavioral Risk Factor Surveillance System and the National Health Interview Survey. Information might also be sought from particular groups at known risk for specific genetic conditions. In all assessments, the needs and concerns of the general public and affected subgroups must be addressed in the design of the research activities.
Thus the process and evaluation of genetic testing requires that CDC and other HHS agencies interact with health care practitioners, public health professionals, laboratory personnel, the general public, and affected consumers. Together, these partners will determine the quality of:
- Pretest counseling and informed consent procedures.
- Test specimens and the security of these specimens.
- Accuracy of analysis.
- Communication of test results to clients.
- Test interpretation.
- Counseling of clients about treatment options.
- Ensuring the confidentiality of test results.
Standards and guidelines
The second main set of activities in the genetic testing program component concerns the development of standards, regulations, and guidelines to ensure the accuracy, validity, and precision of laboratory procedures and to ensure that other quality assurance issues are addressed as well.
All clinical laboratories in the United States that provide information to referring physicians are certified under the Clinical Laboratory Improvement Act (CLIA) Amendments of 1988 (CDC 1992). CLIA standards for quality control, proficiency testing, personnel, and other quality assurance practices apply to all genetic tests. The CLIA regulations, which are jointly developed and administered by the Health Care Financing Administration and CDC, include additional specific requirements for cytogenic testing. The NIH-DOE Task Force on Genetic Testing recently recommended the creation of a genetics subcommittee of the CLIA Advisory Committee to help consider more specific requirements for molecular genetic testing, as well as other improvements and changes as needed.
CDC can also lend its considerable experience in developing model quality assurance programs, including proficiency testing programs in genetic testing in public health programs such as the Newborn Screening Quality Assurance Program (see box). These model programs would set standards for testing, monitor the quality of testing, and recommend improvements for laboratory quality assurance. Through workshops, training, consultation, the development of guidelines, and technology transfer, CDC, in collaboration with other HHS agencies, can work with quality assurance programs operated by state health departments, professional organizations, and public health agencies to ensure high-quality results from all laboratories involved in genetic testing. CDC can draw on its experience from the Model Performance Evaluation Program, the National Laboratory Training Network, and the publication of recommendations, guidelines, and standards for laboratory practice (generally as supplements of CDC's Morbidity and Mortality Weekly Report).
The Task Force acknowledges the proficiency testing program that is run jointly by the American College of Medical Genetics (ACMG) and the College of American Pathologists (CAP), as well as CAP's Laboratory Accreditation Program, both of which are designed to improve the quality of genetic testing. Through regular discussions with these organizations, the role of CDC in quality assurance will be carefully delineated in order to avoid unnecessary duplication of activities.
Since 1978, CDC and its cosponsors, the Health Resources and Services Administration and the Association of Public Health Laboratories, have conducted research on materials development and assisted laboratories with quality assurance in an effort to screen newborns for treatable, inherited metabolic disorders. The program aims to improve the comparability of laboratory results and to standardize the laboratory procedures that use dried blood spot samples for neonatal screening. The program currently serves 78 domestic screening laboratories and 92 laboratories in 28 foreign countries.
|
|
Component 3: Intervention development, implementation, and evaluation
The translation of advances in human genetics into disease prevention opportunities occurs through this program component. As specific genotypes are associated with the development of disease or disability, interventions may be introduced into the health care system, such as the managed care setting, to promote health and reduce the morbidity and mortality from conditions associated with selected genotypes. The role of public health will be to develop strategies for such interventions, implement pilot demonstration programs and evaluate the impact of interventions on reducing morbidity and mortality in the population.
Development
In deciding whether to develop such interventions, several considerations must be taken, including:
- The likelihood of subsequent ill health given the presence of a particular genotype.
- Whether the means for detecting the genotype are valid and cost-effective.
- Whether modification of risk factors can reduce the risk for disease or disability among people who carry specific genotypes.
- Whether interventions can be made available to the people who need them.
- Whether interventions are supported by multiple partners and the public.
Collaboration is particularly important to the design of intervention programs. Key partners are essential to identifying potential ethical or legal concerns, policy constraints, or design flaws. Such flaws might stem from an incomplete understanding of the people to be served and their attitudes toward the accumulation and use of genetic information. Involvement of both consumers and behavioral scientists is important to understanding how potential clients assess risks and benefits, what factors affect clients' compliance with proposed interventions, how to train genetic counselors to deliver these interventions, and how to monitor services.
Implementation
For population-based intervention programs and demonstration projects to be implemented, the infrastructure for program delivery must be in place or developed. Efficient development of this infrastructure calls for coordination of resources, funds, and activities for:
- Training, education, and certification for laboratorians, clinicians, genetic counselors, and other public health personnel.
- Providing laboratory reagents, standards, certifications, or quality assurance programs.
- Developing guidelines for delivering interventions and counseling clients.
- Ensuring that consumers have access to diagnostic tests and treatment.
Evaluation
As genetic tests and services are incorporated into the health care system, evaluation criteria will be developed and applied to determine whether interventions are having the intended effect and which components contribute most to overall effectiveness. Epidemiologic follow-up studies will evaluate intervention impact and outcome indicators--that is, ascertain how effective population-based interventions are in reducing morbidity and mortality, as well as determine whether genetic tests result in loss of insurability, discrimination, or stigmatization. Such studies would also be able to identify gaps in the delivery of and people's access to interventions. Prevention effectiveness studies can evaluate the economic and social impact of interventions and compare alternative initial testing strategies and intervention strategies. Such studies may be particularly important to informed decision making about who should obtain genetic tests and services. For example, post-intervention information may be needed on whether strategies recommended for the general population are appropriate for subgroups whose genetic composition increases their risk for selected conditions. Effectiveness studies might also compare results by test types (for example, DNA-based tests versus biochemical tests) and communication strategies (for example, specific genetic counseling versus general education).
For genetic interventions, evaluation criteria may also be used to assess confidentiality, potential discrimination against people and groups with specific genetic conditions, and to ascertain public perceptions of intervention programs. Although these issues are not always included in evaluation criteria, recent CDC experience with similar issues surrounding HIV prevention programs suggests that evaluating these factors and regularly incorporating the results into intervention activities can increase community support for programs.
Component 4: Communication and information dissemination
The fourth program component concerns the development and application of a comprehensive and coordinated plan for communication between CDC, health professionals, and the general public about advances in human genetics; the use of genetic tests and services; interventions; and the ethical, legal, and social issues related to these topics.
The full scope of such an overarching, multi-year activity can be only hinted at here since the delivery of effective communications will in itself require the development of a strategic plan. Elements of this plan will specify methods for assessing audiences, developing messages, and selecting media for dissemination of messages.
Early on, however, CDC must gain a baseline understanding of both professional and consumer perceptions of and attitudes toward the recent developments in and future expectations for human genetics. This information must be sought from both consumers and professionals and must be immediately reflected in all CDC's activities in genetics and public health so that those activities are not misunderstood by CDC's partners or the public, but rather gain acceptance. Such acceptance would position CDC as a reliable, credible, and trustworthy agency guarding the public interest and recommending the sound and ethical use of new genetic technologies and interventions.
Because the subject of human genetics can be sensitive, effective communication will be key to the success of public health programs involving genetic research results. This could be achieved by building an informal coalition which includes other HHS agencies, professional organizations, consumers, private industry, and state and local health departments to develop and evaluate communication strategies for genetics and public health. Effectiveness of communication will hinge on many factors, including:
- How well communication strategies are coordinated among the various groups.
- Whether the appropriate audiences are targeted to receive messages that result in health promotion and disease prevention.
- Whether the content of messages is accurate, at the appropriate level of technical detail, and conveys the benefits (if any) of genetic testing in a culturally appropriate manner.
- Whether the appropriate mix of media is used for disseminating information.
Particular attention must be given to the audience of health professionals, such as primary care physicians and nurses, who will often serve as a conduit for information and who can help shape widespread attitudes and behaviors. CDC will need to facilitate the sharing of scientific information among health professionals to help ensure that they have timely access to accurate information. Mechanisms such as distance-based interactive meetings, information centers, and electronic communication will be explored.
Issue 1: Partnerships and coordination
The translation of genetic advances into public health action requires an infrastructure that not only supports multiple activities, but also encourages coordination among many partners.
Partnerships
Many public and private agencies are already involved in a wide variety of genetics issues and activities. As CDC expands its work in applying human genetics in health promotion and disease prevention, developing and enhancing collaborative relationships with these public and private partners is critical. These partnerships can (1) improve the quality of CDC's work by incorporating multiple organizational and consumer perspectives and (2) help identify and eliminate duplication of effort.
Partnerships within the Department of Health and Human Services and other federal agencies (such as the Department of Energy, a major sponsor of the Human Genome Project) can be accomplished through coordination of activities, joint funding arrangements, and memoranda of understanding. The Task Force acknowledges, in particular, the leadership of the Maternal and Child Health Bureau (MCHB) of the Health Resources and Services Administration (HRSA) that has been instrumental in sponsoring maternal and child health genetic services at the state, regional, and national levels. CDC will continue to work with MCHB in applying surveillance and epidemiologic methods to evaluate the impact of programs on the health of mothers and children. At the state and local levels, partnerships can be established with various officials (including state genetics coordinators, state epidemiologists, chronic disease epidemiologists, and state health officers) and with the organizations representing state groups regionally and nationally (including the Council for State and Territorial Epidemiologists and the Council for Regional Genetic Networks). Partnerships with professional and private organizations, such as the American Society of Human Genetics, the American Public Health Association, and the National Society of Genetic Counselors, can also be established. Structures such as the Health Promotion Disease Prevention Research Center Program (in which academic research centers collaborate with genome research centers to identify research findings that can be translated into public health practice) may be used as a model.
Some partnerships may not involve the transfer of funds but rather the sharing of information. CDC must rely on external sources, including consumers and expert consultants, for input and feedback on the use of genetics in health promotion and disease prevention. Such consultation, which may be achieved through advisory committees, working groups, and meetings, can help ensure that CDC's activities complement and build on advances in genetic knowledge and technology, and that the general public and affected target groups accept these programs. The nature and extent of external input will vary according to the programmatic priorities in the different centers, institutes, and offices.
Coordination
Current activities in genetics at CDC are diverse and distributed among different organizational units. As activities expand in response to advances in genetics, the competition for resources and the potential for duplication of effort may increase as well. Yet many of the products suggested for each program component can be shared by multiple organizational entities--in fact, to be successful, some products such as community interventions and messages must be coordinated among activities.
Because rapid advances in genetics research will impact multiple programs within CDC, CDC needs to coordinate, prioritize, and encourage internal collaboration on activities in public health and genetics. Through a coordinated focus on genetics, professionals representing the many disciplines required for these activities can provide services CDC-wide. These services can include:
- Coordinating genetics activities throughout CDC and ensuring that these activities are consistent with the policies and priorities of the Department of Health and Human Services.
- Developing guidelines for setting scientific and programmatic priorities in public health genetics.
- Assessing overall needs for professional expertise and capacity in public health genetics.
- Establishing liaisons and partnerships with external agencies, organizations and constituencies.
- Coordinating workshops on cross cutting critical issues involving multiple programs.
- Identifying information resources in genetics.
- Developing communication strategies.
The personnel assigned to facilitate this coordinated focus might also provide technical expertise for designing new methodologies, developing policies and recommendations, conducting training, and disseminating information. At a minimum, this coordinated focus should ensure that CDC can provide public health leadership in genetics.
Issue 2: Ethical, legal, and social issues
Most areas of health research and practice involve issues of individual autonomy, including privacy, confidentiality, and informed consent. When the use of genetic information becomes involved, however, these and related issues become particularly complex, sensitive, and problematic. Information about a person's genotype can provide unprecedented knowledge about his or her health status and that of his or her family. Having this information can provide individuals and organizations with power that can be used for beneficial purposes, such as health promotion and disease prevention, or for practices that are discriminatory, such as withholding health insurance or rejecting candidates for jobs. What constitutes misuse of genetic information is, however, not clear cut in our society; what is clear is that the possible use of genetic information raises a host of ethical, legal, and social issues, many of which invoke fear and mistrust (Hubbard and Lewontin, 1996; Garver and Garver, 1994).
Activities in all program components proposed in this strategic plan must not only demonstrate our awareness of these issues but also our readiness to address them. Below are several examples of issues that must be considered:
- In designing studies--that is, choosing disease outcomes, populations, and data to be collected--researchers must be sensitive to perceived or actual practices that could result in the misuse of information.
- In developing guidelines for genetic testing, program personnel must establish stringent mechanisms for protecting the privacy of individuals and the confidentiality of test results.
- In designing CDC's plan for communication in genetics, communication specialists must anticipate negative attitudes and beliefs and provide information that promotes understanding and allows for informed decisions.
- In outlining the content of professional training, instructional designers must consult with specialists in bioethics to ensure that ethical, legal, and social issues are incorporated into public health curricula.
To equip CDC for addressing these issues, we need to build in-house expertise in the bioethical ramifications of genetics, although that expertise would necessitate interaction with external sources, including NIH's Ethical, Legal, and Social Implications Program, advisory committees, and diverse professionals convened at specialized conferences. Sources for gaining insight into consumers' perspectives are also needed.
CDC must also address ethical, legal and social issues associated with the use of genetic information by keeping abreast of and advocating for policies, procedures, regulations, and other legal mechanisms that can provide some guidelines (see Appendix I). However, such questions must be continually revisited and the regulatory framework repeatedly adjusted to allow for, prohibit, safeguard, or otherwise control activities in human genetics. Further, many existing laws and regulations should be reviewed for their applicability to the bioethical issues of human genetics.
Issue 3: Education and training
Although many health promotion and disease prevention activities that use genetic information have been ongoing for some time, the field of human genetics continues to expand rapidly. This expansion strains the ability of groups such as public health researchers, health practitioners, policymakers, medical students, and consumers to keep abreast of new information and its potential ramifications. Therefore, systematic and ongoing education and training are needed in order to provide varied audiences, especially public health professionals, with the knowledge and skills necessary to transform the proposed program components into ongoing public health activities. The task force supports the recent development of the National Coalition for Health Professional Education in Genetics, which is led by the American Medical Association and the American Nursing Association and which has representatives from numerous organizations. CDC has an active partnership with this coalition and will continue to emphasize public health perspectives in genetics training.
To accelerate training in genetics and public health for public health professionals, training specialists might research current and upcoming educational opportunities sponsored by various agencies and organizations. The content of courses, workshops, and other educational formats can be assessed and supplemented by new or revised curricula or course work. For example, training for laboratory personnel will need to cover new genetic tests and reagents and may result in recertification of these professionals.
Training modules that address each component of the proposed framework (public health assessment and evaluation of genetic testing, interventions, and communication) will need to be incorporated into training for the Epidemic Intelligence Service. Curricula for public health, medical, nursing, and law schools will need to be adapted so that as new professionals enter the workforce they will have already attained a minimum level of competence in not only genetics and disease prevention but the ethical, legal, and social issues related to it.
The general public will need to be similarly educated so that they become informed consumers of professional services and remain alert to any potential misuse of genetic information. In addition, incorporating information on genetics into the life science curricula of elementary and secondary schools will help prepare the next generation of adults to respond to advances in genetic technology. Moreover, people who will develop the content of courses and communicate with the different audiences will themselves need to become conversant with concepts, terminology, and issues in genetics and have sources available for continual re-education.
To facilitate the rapid transfer of new information, we may need to use mechanisms that go beyond those generally used in standard training. These mechanisms may include providing educators with online access to curriculum modules; establishing relationships with partners, such as prevention research centers, whose own network of partners reaches far into communities; and sponsoring fellowships and residency programs focused on the potential use of genetic technology in preventing disease.
Thus the content, format, length, medium, and other aspects of training will vary markedly by audience and purpose. To ensure that all aspects of this multifaceted education and training endeavor are appropriately addressed, we may need to design an overall plan and then allocate its elements among the program components.
The task force identified seven broad goals that collectively form a strategy for strengthening CDC's existing activities and developing new initiatives in genetics and public health. Each goal is designed to advance CDC's capacity in one or more of the essential program components and cross-cutting areas of critical concern.
Goal 1: Foster partnerships and coordination of genetic activities within and outside of CDC to promote heath and prevent disease and disability.
Objectives:
1.1. Ensure a CDC work environment that fosters coordination of program activities in genetics.
1.2. Assess the resources and expertise available and determine what additional resources and expertise are needed to conduct program activities in genetics.
1.3. Develop, implement, and evaluate a system for establishing scientific and program priorities for genetic activities at CDC.
1.4. Enhance existing partnerships and establish new collaborative relationships in the area of genetics.
1.5. Seek regular external consultation and input on the use of genetics in the promotion of health and the prevention of disease and disability.
Goal 2: Ensure that ethical, legal and social issues are addressed in applying genetics to the promotion of health and the prevention of disease and disability.
Objectives:
2.1. Conduct ongoing reviews of ethical, legal, and social issues in genetics at CDC and the public health community.
2.2. Develop guidelines and standards for the ethical application of genetics in public health research and practice.
2.3. Enhance training programs for CDC and other public health professionals that address ethical, legal, and social issues related to genetic applications in public health research and practice.
Goal 3: Assess how risk for disease and disability is influenced by the interaction of human genetic variation with modifiable risk factors.
Objectives:
3.1. Assess the impact of various human genetic factors on the risk for disease and disability.
3.2. Evaluate gene-environment interactions to identify modifiable risk factors.
Goal 4: Ensure the appropriateness and quality of population-based genetic testing.
Objectives:
4.1. Evaluate the appropriateness of genetic tests for use on a population basis.
4.2. Support a system of laboratory quality assurance.
4.3. Assess the access to, use of, and impact of population-based genetic testing.
Goal 5: Ensure that genetic tests and services are incorporated in population-based interventions that promote health and prevent disease and disability.
Objectives:
5.1. Develop population-based interventions that use selected genetic tests and services to promote health and prevent disease and disability.
5.2. Evaluate the impact that the use of selected genetic tests and services has on the promotion of health and the prevention of disease and disability.
Goal 6: Build capacity to promote health and prevent disease and disability by training public health professionals in genetics.
Objectives:
6.1. Enhance health professionals' knowledge of the role of genetics in promoting health and preventing disease and disability.
6.2. Build a scientific core of personnel trained in genetics and public health.
6.3. Provide continuing education opportunities and specialized training for health professionals and people in related fields.
Goal 7: Provide timely and accurate information to both the general public and professional audiences on the role of genetics in the promotion of health and the prevention of disease and disability.
Objectives:
7.1. Increase public awareness and understandig of the role of genetics in disease prevention so that people may make informed decisions about health behaviors and genetic testing and services.
7.2. Facilitate scientific information sharing to ensure that health professionals and those in related fields have access to timely and accurate information on genetics and disease prevention.
Introduction
The recommendations presented here are the initial action steps of the strategic plan. These recommended actions are an outgrowth of the goals and objectives previously described. Consistent with the philosophical foundation of the strategic plan, the recommendations are designed to ensure that the highest ethical standards are used in applying knowledge in human genetics to promoting health and preventing disease and disability.
The recommendations are presented in two parts: immediate actions for fiscal years 1997-1998 and continued actions for fiscal years 1999-2001. Both parts should be viewed as integrated sets of activities that create the overall environment necessary for a successful endeavor. Further, the recommendations are not necessarily listed in order of priority since selected activities must be simultaneously undertaken in multiple areas. The implementation plan for these recommendations will take into account the availability of resources.
Recommendations for immediate action
1. Sustain a coordinated focus on genetics and public health at CDC.
The program activities recommended in this strategic plan will be implemented by CDC's various centers, institutes, and offices. A CDC
National Office of Public Health
Genomics should perform the following functions: (1) coordinate genetics activities throughout CDC and ensure that these activities are consistent with the policies and priorities of the Department of Health and Human Services; (2) develop guidelines for setting cross-cutting priorities in public health genetics; (3) assess needs for professional expertise and capacity for activities in genetics at CDC and at the state level; (4) cultivate partnerships with external agencies, organizations, and constituencies to coordinate efforts and ensure a productive exchange of information and concerns; (5) assess how current and proposed laws, regulations, and rulings affect genetics activities at CDC and the state level (in collaboration with CDC's Office of the Director); and (6) coordinate workshops on critical cross-cutting issues (e.g., informed consent), from which guidelines and recommendations may emerge.
2. Establish mechanisms for external input, particularly for ethical, legal, and social issues.
Create a liaison with the Ethical, Legal, and Social Implications Program of the National Institutes of Health's National Human Genome Research Institute. Consider establishing a genetics subcommittee of the CDC Advisory Committee and ensure that these efforts are coordinated with other HHS advisory committees on genetics. Convene external consultants from different organizations to discuss early implementation steps of this strategic plan and seek regular external input thereafter. These mechanisms can help ensure that CDC remains connected with and responsive to a broad community that can help anticipate and address the complex philosophical and practical issues of genetics at CDC.
3. Provide public health professionals with training opportunities to enhance their skills and knowledge.
Develop an introductory training course in genetics and public health that includes a module on ethical, legal, and social issues. Sponsor a CDC-wide career development program to develop additional expertise in genetics at CDC and the public health community. Develop a network of professionals involved in epidemiologic studies of human genes to foster collaboration, information sharing, and training. These educational activities will help public health professionals better understand the role of genetics in disease prevention.
4. Develop a strategy for communication about genetics.
In collaboration with CDC's Office of Communication, conduct a comprehensive review of communication research in genetics, develop a plan for assessing the information needs of various audiences, develop messages, and select media for disseminating information about genetics and public health. Use the Internet as one distribution mechanism. These activities will ensure that the dissemination of information is coordinated, accurate, and timely.
5. Sponsor intramural activities on applied genetic research.
Make seed money available, through a competitive funding process, to support startup genetics projects throughout CDC. Available funding from CDC can be supplemented with funds from the different centers, institutes and offices. Establish a panel for the objective review and ranking of proposals according to agreed upon criteria. These projects will allow CDC to expand ongoing activities and build scientific credibility and capacity for new activities in multiple centers, institutes, and offices.
6. Expand activities to ensure the quality of genetic testing.
Establish a genetics subcommittee for the advisory committee of CLIA (Clinical Laboratory Improvement Act Amendments of 1988). In collaboration with the Food and Drug Administration and the Health Care Financing Administration, improve CLIA regulations by creating a new category for molecular diagnostics. Collaborate with professional organizations such as the American College of Medical Genetics and the College of American Pathologists to define CDC's role in quality assurance for genetic testing in relation to that of other organizations. Conduct studies on methods to improve testing performance and to develop guidelines and models for quality assurance.
7. Sponsor extramural projects to evaluate intervention programs that use genetics tests and services.
Use a competitive process to evaluate ongoing interventions (e.g., cell disease, or familial hypercholesterolemia) and assess prevention effectiveness. Support for extramural activities should focus on applied research in diverse populations and various settings. The amount of funding will depend on availability of resources.
8. Sponsor the first annual meeting on genetics in public health.
Create a forum for the ongoing exchange of information on the application of genetic advances to public health practice. Sessions can cover recent discoveries, ethical concerns, data sources, programmatic considerations, policy issues, information technology, and other topics. Professionals from various disciplines can share their experiences and concerns about the effect of genetic advances on public health.
Recommendations for continued action (FY 1999-2000)
1. Extend training opportunities in genetics and public health.
Offer continuing education and specialized training in laboratory methods and genetic epidemiology for CDC personnel and others involved in genetic research or service delivery. Provide support to academic institutions and health departments for developing the capacity of public health professionals in genetics and disease prevention. These activities strengthen the public health community by helping it keep pace with continued advances in genetics and be prepared to address new issues.
2. Cosponsor the development of an integrated genetics information network.
Collaborate with the National Coalition for Health Professional Education in Genetics and other public and private organizations in developing a virtual information center for genetics. Make the system accessible to health professionals and the general public but target components of it to specific audiences. Use the most advanced information technologies to interconnect users, information sources, and services so that they can better exchange information.
3. Expand existing surveillance and health information systems for the collection and analysis of genetics data.
Enhance existing systems designed to determine the population frequency of selected health conditions and genotypes and to gain information on the potential public health impact of genetic testing. In collaboration with HHS agencies, develop model surveillance systems for rare genetic diseases that have a collective effect on public health. Together, these activities will advance knowledge about the interaction between genotype, environment, and disease, and provide information crucial to the ongoing setting of priorities, allocation of resources, and development of programs.
4. Expand activities to ensure the quality of genetic testing programs.
Add to the activities recommended for immediate action by (1) developing model quality assurance programs for selected genetic tests and technologies and (2) sponsoring population-based studies of genetic testing issues. Ensure that these activities assess not only laboratory testing but genetic counseling services, ethical concerns, people's access to genetic testing, and the validity of test results. Determine how valuable the use of specific genetic tests are to reducing death, disease, and disability.
5. Develop guidelines and recommendations on the use of genetic tests and services.
In collaboration with HHS agencies, issue recommendations on population-based testing for selected conditions and guidelines for guarding against the misuse of genetic information. This information will provide both practitioners and consumers with information needed to make informed decisions. These activities are consistent with CDC's role in providing guidance in health promotion and disease prevention.
6. Sponsor extramural projects to assess interventions based on genetic tests and services.
Support community-based demonstration projects that integrate interventions using genetic tests and services into public health practice. Develop partnerships between public health and academic research centers that will translate the findings from the Human Genome Project into health promotion and disease prevention strategies. Such centers will enhance the network through which research results can reach people in communities.
Continual advances in human genetics will offer new opportunities for disease prevention and health promotion and may lead to a new paradigm for individualized preventive medicine. This strategic plan lays out the long-term vision for the role of public health in translating advances in human genetics into health promotion and disease prevention. Public health professionals will have an important role in assessing the public health impact of gene-environment interaction and the health needs of various populations; in ensuring the appropriateness and the quality of genetic tests and services; in evaluating the impact of genetic tests and services on the health of the populations; and in addressing complex ethical, legal, and social issues. The goals of the strategic plan for genetics are the foundation for immediate, continued, and not-yet-defined actions necessary to translate advances in human genetics into public health action.
- Centers for Disease Control and Prevention. Regulations for implementing clinical laboratory improvement amendments of 1988: A summary. MMWR 1992;41:RR-2;1-17.
- Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CRK5 structural gene. Science 1996;273:1856-61.
- Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary hemochromatosis. Nat Genet 1996;13:399-408.
- Garver KL, Garver B. The Human Genome Project and eugenic concerns. Am J Hum Genet 1994;54:148-58.
- Guyer MS, Collins FS. How is the Human Genome Project doing and what have we learned so far? Proc Natl Acad Sci U S A 1995;92:10841-8.
- Hoffman EP. The evolving genome project: Current and future impact. Am J Hum Genet 1994;54:129-36.
- Hubbard R, Lewontin RC. Pitfalls of genetic testing. N Engl J Med 1996;334:1192-4.
- Institute of Medicine. The future of public health. Washington, DC: National Academy Press, 1988.
- Juengst ET. "Prevention" and the goals of genetic medicine. Hum Gene Ther 1995;6:1595-605.
- Khoury MJ, Beaty TH, Cohen BH. Fundamentals of genetic epidemiology. New York: Oxford University Press, 1993.
- Khoury MJ, Genetics Working Group. From genes to public health: applications of genetics in disease prevention. Am J Public Health 1996;86:1717-22.
- Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994;266:66-76.
- Omenn GS. Genetics and public health. Am J Public Health 1996;86:1701-4.
- Online Mendelian Inheritance in Man™, Baltimore, MD: Center for Medical Genetics, Johns Hopkins University, and Bethesda, MD: National Center for Biotechnology Information, National Library of Medicine, 1997 [database].
- Satcher D. CDC's first 50 years: lessons learned and relearned. Am J Public Health 1996;86:1705-8.
- Watson JD. The Human Genome Project: Past, present, and future. Science 1990;248:44-9.
- Whelan WJ. Genetics, ethics, and human values. FASEB J 1995;9:699-700.
Appendix A: Hereditary hemochromatosis and iron overload diseases
- Hereditary hemochromatosis is a genetic disorder of iron metabolism that increases iron absorption and results in lifelong excessive iron accumulation in the body.
- Hereditary hemochromatosis affects 1 in 400 to 1 in 200 people, and 1 in 10 people is a carrier, which makes the condition the most common known genetic disorder.
- In affected people, excessive iron deposits may result, usually after age 40, in arthritis, cirrhosis of the liver, diabetes, impotence, and heart failure.
- A major gene for hemochromatosis was cloned in 1996. The gene is linked with the major histocompatibility complex region on chromosome 6.
- Lifetime risk for disease is not well defined. Estimates range from 20% for women to more than 80% for men.
- Iron intake in the diet is the interacting factor. Interaction may also occur with infectious agents, alcohol, vitamin C, and other hereditary conditions (such as porphyria).
- Periodic phlebotomy throughout life is a very effective, life-saving intervention.
- Elevated transferrin saturation (TS) is a good screening test for iron overload. The sensitivity of the test is about 90%, and its positive predictive value is 10% to 20%.
- Important public health issues for discussion include whether or not to do population-based screening using TS or a DNA-based test and in which groups, whether to conduct population studies to assess disease risks, how to assess prevention effectiveness of screening, how to educate people about this condition, how to protect people from discrimination, and whether to use blood donated by people with this condition.
- In March 1997, CDC, in collaboration with National Human Genome Research Institute, held an expert panel meeting to assess the impact of gene discovery on the diagnosis, management, and prevention of iron overload associated with hereditary hemochromatosis. A summary of this meeting is being drafted for publication.
Appendix B: Hereditary breast cancer in BRCA1 carriers
- About 3% to 5% of breast cancers occur in women who carry mutations of BRCA1. Breast cancer in these carriers has not been shown to be histologically different from sporadic breast cancer. But women who carry these mutations often get cancer earlier and have multiple primary cancers more often than do women with sporadic cancer. Multiple family members get breast cancer in these families, and cancer develops in other sites, such as the ovaries.
- BRCA1 mutations are believed to occur in about 1 in 500 people.
- Breast cancer in BRCA1 mutation carriers is more likely to occur at a younger age than sporadic breast cancer. BRCA1 mutations increase women's risk for ovarian cancer, and to a lesser degree, men's risk for for prostate cancer.
- BRCA1 was cloned in 1994 on chromosome 17q. As of 1996, more than 150 mutations had been found in BRCA1. Only about 11 mutations have been found in more than two families.
- In studies of high-risk families, lifetime risk for breast cancer among BRCA1 carriers has been estimated at 50-85%, and the risk for ovarian cancer at about 15-40%.
- Little information is available on how BRCA1 interacts with other breast cancer risk factors.
- The efficacy of prophylactic mastectomy for preventing breast cancer has not been established. Prophylactic oophorectomy is believed to decrease risk for ovarian cancer, but a percentage of women with a family history of ovarian cancer have developed abdominal carcinomatosis after prophylactic oophorectomy. Screening of young women by using mammography, transvaginal ultrasound, or CA-125 concentration has not been proven to save lives.
- Tests are commercially available for BRCA1 mutations, but the risks of testing (psychological distress, insurance discrimination, employment or relationship problems) may outweigh the benefits (relief from anxiety if the test is negative, certainty about medical decisions or psychologic adjustment if the test is positive).
- Important public health issues for discussion include whether the test should be marketed to the general public in the absence of patient counseling services, the penetrance and prevalence of BRCA1 mutations, and the effectiveness of proposed interventions, such as prophylactic surgery and screening for CA-125. Other ethical and social issues about testing in the absence of a proven intervention also need to be addressed.
Appendix C: The importance of a new chemokine receptor allele (CCR5 D32) in HIV infection
- CCR5 is a chemokine receptor on cell surfaces. It binds cell-signaling molecules called chemokines (e.g., RANTES, MIP-1a, and MIP-1b). This interaction results in the activation and movement of immune cells to sites of infection, leading to enhanced clearance of pathogens.
- HIV, the virus that causes AIDS, has usurped this normal cellular receptor and uses it to enter immune cells.
- The CCR5 receptor occurs in at least two forms, wild type (W) and deleted (D32). The deletion of 32 base pairs of DNA in the CCR5 gene results in a CCR5 receptor that does not get to the cell surface.
- People with two copies of the deleted CCR5 receptor (D32/ D32), while appearing to have no immune system abnormalities, may be strongly protected from HIV infection. This is because the virus can no longer enter some immune system cells, specifically those most implicated in HIV transmission and early infection.
- Because HIV can enter immune cells by using other cell surface receptors, not all people with the D32/D32 CCR5 genotype are likely to be protected from HIV infection. To date, only one person with two copies of the deleted CCR5 gene has been reported to be HIV positive.
- People with HIV infection who have one copy of the deleted gene (W/D32 CCR5 genotype) may not develop AIDS as quickly as do people who have the wild type allele (W/W) only.
- The D32 CCR5 allele is found predominantly in people of European descent. So far it has not been found in people from parts of Asia and Africa. Homozygosity for the D32 CCR5 allele is found in about 1% of white people, and heterozygosity is found in 10% to 20%. Three to four percent of black people have the heterozygous (W/D32) genotype.
- The D32 CCR5 allele is easily detected by using PCR and restriction digest techniques. However, genetic testing for CCR5 allele status remains a research tool because protection associated with having two copies of the D32 allele appears not to be complete. It is not yet known whether testing for the heterozygous state (W/D32) is truly predictive of outcomes among people with HIV/AIDS.
- In response to questions from the public about these new findings, CDC and other HIV/AIDS research organizations prepared public health messages. These messages emphasized the need to continue proven methods for reducing exposure to HIV regardless of CCR5 genotype (CDC HIV/AIDS Prevention Newsletter, February 1997: 1-2 ).
- The data about CCR5 and HIV have stimulated the development of potential therapies for the prevention and treatment of HIV infection.
Appendix D: Examples of CDC activities in genetics
I) Public health assessment
Surveillance
- Birth defects and developmental disabilities
- Hemophilia
Molecular and genetic epidemiology
- Genetic risk factors for spina bifida
- CCR5, HLA, and other immune system genes in HIV+ and HIV- populations
- HLA genes in people with measles, hantavirus, human papillomavirus, malaria, coccidiomycosis, rheumatoid arthritis, and cervical neoplasia
- DNA adducts, metabolic enzyme polymorphisms, HLA, oncogenes, and other genetic biomarkers in people with occupational diseases
- Vitamin D receptor alleles and osteoporosis risk
- HLA-H and disease in people with hemophilia
- Thrombosis and CVS-related genes in people with hemophilia or people with vascular disease
Other
- NHANES DNA bank: Establishment of cell lines from a representative U.S. sample
- Assessment of fetal risk from chorionic villus sampling
- Assessment of newborn screening for cystic fibrosis (workshop)
II) Evaluation of genetic testing
- Newborn Screening Quality Assurance Program
- CLIA 88 regulations
III) Intervention development, implementation, and evaluation
- National program to prevent iron overload
- Evaluation of reduction of morbidity following newborn screening for sickle cell disease
- Prevention of joint disease in people with hemophilia
- Prevention of factor VIII inhibitor formation in people with hemophilia
IV) Communication and information dissemination
- CD-ROM project on the genetic basis of cancer
- CDC statements on hemochromatosis, CCR5, and HIV infection
- Electronic communications on news in genetics and disease prevention
Appendix E: CDC Task Force on Genetics in Disease Prevention
- Rob Anda, M.D., National Center for Chronic Disease Prevention and Health Promotion
- Timothy Baker, B.S., National Immunization Program
- Carol Boussy, Ph.D., National Center for Environmental Health
- Joanne Cono, M.D., M.Sc. National Center for Environmental Health
- Gayle DeBord, Ph.D., National Institute for Occupational Safety and Health
- Julie Fishman, M.P.H., National Center for Chronic Disease Prevention and Health Promotion
- Gale Gardiner, Ph.D., National Center for Health Statistics
- Wayne Giles, M.D., M.S., National Center for Chronic Disease Prevention and Health Promotion
- Barbara Gray, M.Ln., National Center for Chronic Disease Prevention and Health Promotion
- Harry Hannon, Ph.D. National Center for Environmental Health
- Dick Keenlyside, M.D., Public Health Practice and Program Office
- Muin Khoury, M.D., Ph.D., National Center for Environmental Health
- Ellen King, National Center for Environmental Health
- Paula Kocher, J.D., Office of the Director
- Deena Koniver, M.S., Office of the Associate Director for Science
- Janet McNicholl, M.D., National Center for Infectious Diseases
- Leslie O'Leary, Ph.D., National Center for Environmental Health
- Fred Rickles, M.D., National Center for Infectious Diseases
- Dawn Smith, M.D., National Center for HIV/AIDS, STD, and TB Prevention
- Karen Steinberg, Ph.D., National Center for Environmental Health
- Deborah Tress, J.D., Office of the Director
- Ben Truman, M.D. Epidemiology Program Office
- Diane Wagener, Ph.D., National Center for Health Statistics
- Dometa Williams, National Center for Environmental Health
Appendix F: Meeting participants, Translating Advances in Human Genetics into Public Health Action, January 27-28, 1997
Name, Organization, & Affiliation
- Thom Berry, South Carolina Department of Health and Environmental Control; National Public Health Information Coalition
- Karina Boehm, MPH, National Human Genome Research Institute
- Donna Brown, Research Genetics
- James Cheek, MD, MPH, Indian Health Service
- Roberta Crawford, Iron Overload Diseases Association
- George Cunningham, MD, California Department of Health Services
- Mary Davidson, MSW, Alliance of Genetic Support Groups
- Franklin Desposito, MD, UMDNJ - New Jersey Medical School, Department of Pediatrics; American Academy of Pediatrics
- Louis J. Elsas II, MD, Emory University Department of Pediatrics, Medical Genetics Pediatrics; Council of Regional Genetic Networks
- Tom Frank, MD, Myriad Genetics, Inc.
- John Gallicchio, Health Resource and Service Administration, Maternal and Child Health Bureau
- Rani George, MPH, Association of Asian Pacific Community Health Organizations
- Wayne Grody, MD, PhD, UCLA School of Medicine, Division of Medical Genetics and Molecular Pathology; College of American Pathologists
- Steve Groft, PharmD, National Institute of Health, Office of Rare Diseases
- Joseph Hackett, MD, Food and Drug Administration
- Arthur Hackman, National Hemophilia Foundation
- James W. Hanson, MD, National Institute for Child Health Development
- Stanley Inhorn, MD, Wisconsin State Laboratory of Hygiene; Association of Public Health Laboratories
- Fatimah Jackson, PhD, University of Maryland, Department of Applied Biological Anthropology Research; Human Biology Association
- Michael Katz, MD, March of Dimes
- Heidi L. Keller, Office of Health Promotion, Washington Department of Health; Association of State and Territorial Directors of Health Promotion and Public Health Education
- Michael Knapp, National Center for Genome Resources
- Michael Langan, National Organization for Rare Disorders
- David Lanier, MD, Agency for Health Care Policy and Research
- Linda R. Lebovic, MT, Health Care Financing Administration, Health Standards and Quality Bureau
- Eugene Lengerich, VMD, North Carolina Department of Health, Division of Health Promotion; Council of State and Territorial Epidemiologists
- Glenn McGee, PhD, University of Pennsylvania, School of Medicine, Center for Bioethics
- Patricia Murphy, PhD, GeneWise
- Daniel W. Nebert, MD, University of Cincinnati Medical Center
- Pat J. Numann, MD, State University of New York, College of Medicine, Health Science Center; American Medical Association
- Victoria Odesina, RN, Sickle Cell Service, Gengras Ambulatory Center, Sickle Cell Service
- Reed Pyeritz, MD, PhD, Allegheny General Hospital, Department of Human Genetics; American College of Medical Genetics
- Ana Rivas Beck, JD, National Coalition of Hispanic Health and Human Services Organizations
- Mark Rothstein, JD, Health, Law and Policy Institute, University of Houston
- Sheldon Samuels, Ramazzini Institute
- Katherine Schneider, Dana-Farber Cancer Institute; National Society for Genetic Counselors
- Morton Schwartz, PhD, Memorial Sloan-Kettering Cancer Center; CLIAC Genetics Committee
- Stephanie Sherman, PhD, Emory University School of Medicine, Department of Genetics and Molecular Medicine; American Society of Human Genetics
- Brad Therrell, PhD, Texas Department of Health, Chemical Services Division; International Society for Neonatal Screening
- Elizabeth Thomson, National Human Genome Research Institute
- Martin Wasserman, MD, JD, Maryland Department of Health and Mental Hygiene; Association of State and Territorial Health Officials
- Robert Weir, PhD, Program in Biomedical Ethics and Medical Humanities, University of Iowa
- Ann M. Willey, PhD, Division of Laboratory Quality Certification, New York State Department of Health
- Kathleen Zeitz, JD, National Breast Cancer Coalition
Appendix G: Summary of conclusions reached at "Translating Advances in Human Genetics into Public Health Action," a meeting held January 27-28, 1997
- Participants expressed enthusiastic support for CDC's role in genetics and public health.
- High expectations exist for CDC and the need to set priorities for activities.
- Coordination with other federal agencies is essential.
- Partnerships with numerous stakeholders are necessary.
- Ethical issues are the greatest challenge.
- Communication, education, and community involvement are important.
- CDC's greatest strengths are epidemiology, surveillance, and population-based data collection.
- Needs assessments should be conducted both internally and at the state level.
- A clearinghouse for genetics information related to public health is needed.
- Support for CDC's role in laboratory quality assurance and proficiency testing was expressed.
- Diseases of low frequency and high severity should not be ignored.
Appendix H: Selected recommendations* of the joint NIH-DOE Task Force on Genetic Testing
- The Secretary of Health and Human Services should create an advisory committee on genetic testing involving multiple organizations and federal agencies.
- Institutional Review Boards must ensure that protocols for the development of genetic tests can be used predictively when subject identifiers are retained and when the tests are to be readily available for clinical use.
- Highlight CDC's role in facilitating the collection of data on the safety and effectiveness of new genetic tests. "CDC should play a coordinating role in data gathering and should be allocated sufficient funds for this purpose. CDC's role is particularly important in collecting data in normal populations, e.g., on disease-related allele frequencies and in collecting data from multiple sources to facilitate review of new tests, particularly for rare diseases. The Task Force welcomes recent CDC initiatives to (1) expand its population-based surveillance systems in order to provide data on the validity of genetic tests and post-test interventions, and (2) conduct follow-up epidemiologic studies on individuals tested for specific genotypes to learn more about test validity, the natural history of genetic disorders, and the safety and effectiveness of intervention. These efforts should be in collaboration with other Federal and State agencies and private organizations."
- Genetic test developers should submit validation and clinical utility data to external review as well as to interested professional organizations in order to permit users to make informed decisions about routine use.
- "The Task Force urges the newly created genetics subcommittee of the Clinical Laboratory Improvement Advisory Committee to consider the creation of a specialty of genetics which would encompass all predictive tests that satisfy criteria for stringent scrutiny. If only a subspecialty for DNA/RNA-based tests is feasible, the subcommittee must then address how to assure the quality of laboratories performing nonDNA/RNA predictive genetic tests. The agencies primarily responsible for administering CLIA, HCFA, and CDC should take the lead in implementing this recommendation."
* The Final report of the Task Force on Genetic Testing: Promoting Safe and Effective Genetic Testing in the United States - Principles and Recommendations is available on the world wide web at http://www.genome.gov/page.cfm?pageID=10001733.
Appendix I: Examples of ethical issues crucial to public health genetics programs
Assuming that knowledge about genetic risk factors can be used to prevent morbidity and mortality, ethical issues include 1) voluntariness of programs, 2) informed consent, 3) disclosure of results of genetic testing, 4) privacy concerns in large scale surveillance programs, and 5) concerns about group stigmatization. The following are examples of specific questions that may arise:
- How can truly informed consent for genetic testing be obtained in public health practice (versus clinical practice, in which personal contact and the opportunity for one-on-one interaction is greater)? How does informed consent for genetic testing in public health practice differ from informed consent for other public health services?
- Should counseling services be provided as part of public health practice even when risks associated with different genetic polymorphisms and mutations vary widely? How can such services be provided?
- How can privacy and confidentiality be maintained in the public health setting?
- How should population-based disease registries be handled? What are their immediate and long-term benefits? What are their immediate and long-term risks? How can public health agencies maximize benefits and minimize the risks associated with such registries?
- Under what circumstances, if any, is it appropriate to make specimens anonymous?
- Are there ethical alternatives to classic reporting* for public health practice, especially when large populations are involved?
- What rules should exist for making specimens anonymous or destroying specimens obtained in public health practice?
- Under what circumstances is new consent for archived specimens needed for public health investigations?
- How can consent be tracked on specimens from many sources, including hospital laboratories?
- Do situations occur in public health when genetic testing could be done on identifiable specimens without informed consent?
*Classic reporting refers to the one-on-one interaction between a patient and a doctor, nurse, or counselor for explaining a genetic test and its results. An alternative might be to tell all participants that an equal number of those who have and those who do not have a specific mutation will be contacted for post-test counseling. In this way, people who receive a call cannot assume what their results are. |


