What is pH?
Acidic and basic are two extremes that describe chemicals, just like hot and cold are two extremes that describe temperature. Mixing acids and bases can cancel out their extreme effects; much like mixing hot and cold water can even out the water temperature. A substance that is neither acidic nor basic is neutral.
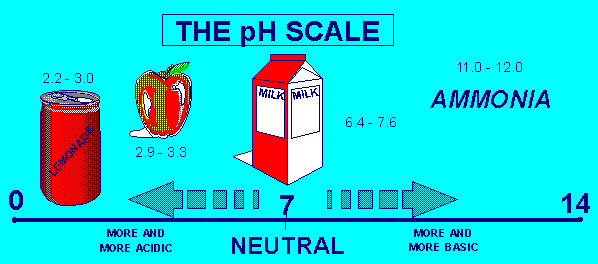
The pH scale measures how acidic or basic a substance is. It ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic, and a pH greater than 7 is basic. Each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times (10 times 10) more acidic than a pH of 6. The same holds true for pH values above 7, each of which is ten times more alkaline—another way to say basic—than the next lower whole value. For example, a pH of 10 is ten times more alkaline than a pH of 9.
Pure water is neutral, with a pH of 7.0. When chemicals are mixed with water, the mixture can become either acidic or basic. Vinegar and lemon juice are acidic substances, while laundry detergents and ammonia are basic.
Chemicals that are very basic or very acidic are called “reactive.” These chemicals can cause severe burns. Automobile battery acid is an acidic chemical that is reactive. Automobile batteries contain a stronger form of some of the same acid that is in acid rain. Household drain cleaners often contain lye, a very alkaline chemical that is reactive.
The following diagram shows the pH scale and the pH of some common items:
![[logo] US EPA](https://webarchive.library.unt.edu/eot2008/20081027193705im_/http://www.epa.gov/epafiles/images/logo_epaseal.gif)
